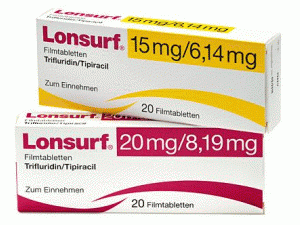
复方曲氟尿苷盐酸/替吡嘧啶薄膜片Lonsurf 15mg/6.14mg Filmtabletten
 药店国别:
产地国家:德国
处方药:是
所属类别: 15毫克/6.14毫克/片 20片/盒
包装规格: 15毫克/6.14毫克/片 20片/盒
计价单位:盒
生产厂家中文参考译名:
生产厂家英文名:SERVIER Deutschland GmbH
原产地英文商品名:Lonsurf 15mg/6.14mg Filmtabletten 20Stk
原产地英文药品名:Trifluridine/Tipiracil Hydrochloride
中文参考商品译名:Lonsurf复方薄膜片 15毫克/6.14毫克/片 20片/盒
中文参考药品译名:曲氟尿苷盐酸/替吡嘧啶
药店国别:
产地国家:德国
处方药:是
所属类别: 15毫克/6.14毫克/片 20片/盒
包装规格: 15毫克/6.14毫克/片 20片/盒
计价单位:盒
生产厂家中文参考译名:
生产厂家英文名:SERVIER Deutschland GmbH
原产地英文商品名:Lonsurf 15mg/6.14mg Filmtabletten 20Stk
原产地英文药品名:Trifluridine/Tipiracil Hydrochloride
中文参考商品译名:Lonsurf复方薄膜片 15毫克/6.14毫克/片 20片/盒
中文参考药品译名:曲氟尿苷盐酸/替吡嘧啶
简介
部份中文曲氟尿苷盐酸/替吡嘧啶处方资料(仅供参考) 中文名称:复方曲氟尿苷盐酸/替吡嘧啶片/TAS-102 药品名称:Trifluridine+Tipiracil 商品名:Lonsurf 剂型:复方药片剂型 给药途径:口服药 国外批准日期:2015/9/22 作用机制: LONSURF 作用机制 核苷代谢抑制剂trifluridine与胸苷磷酸化酶抑制剂tipiracil复方制剂,干扰DNA合成,抑制细胞增殖 适应症: Lonsurf是一种口服药,适用于既往接受过化疗和生物疗法的晚期(转移性)结直肠癌患者。 剂量和给药方法 ⑴ 推荐剂量:35mg/m2/dose口服每天2次在每28天疗程第1至5天和第8至12 of天。 ⑵ 早晨和傍晚餐完成后1小时内服用LONSURF。 常见不良反应: 贫血、抗感染白细胞减少(中性粒细胞减少症)或血小板减少(血小板减少症)、身体虚弱、极度疲劳和乏力、恶心、食欲减退、腹泻、呕吐、腹痛和发烧。既往接受过化疗和生物疗法的晚期(转移性)结直肠癌患者的新选择。 临床试验获益: 结直肠癌:一项入组800名经治转移性结直肠癌患者的国际随机双盲临床试验评估了Lonsurf的有效性与安全性。平均而言,Lonsurf治疗组患者的总生存期和无进展生存期分别是7.1个月和2个月,相比之下,安慰剂治疗组患者的总生存期和无进展生存期分别只有5.3个月和1.7个月。复方曲氟尿苷盐酸/替吡嘧啶薄膜片英文版说明书
IMPORTANT SAFETY INFORMATIONWARNINGS AND PRECAUTIONSSevere Myelosuppression: LONSURF caused severe and life‑threatening myelosuppression (Grade 3‑4) consisting of neutropenia (38%), anemia (18%), thrombocytopenia (5%), and febrile neutropenia (3%). Two patients (0.2%) died due to neutropenic infection. A total of 12% of LONSURF‑treated patients received granulocyte‑colony stimulating factors. Obtain complete blood counts prior to and on day 15 of each cycle of LONSURF and more frequently as clinically indicated. Withhold LONSURF for febrile neutropenia, absolute neutrophil count less than 500/mm3, or platelets less than 50,000/mm3. Upon recovery, resume LONSURF at a reduced dose as clinically indicated.Embryo‑Fetal Toxicity: LONSURF can cause fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to the fetus. Advise females of reproductive potential to use effective contraception during treatment and for at least 6 months after the final dose.USE IN SPECIFIC POPULATIONSLactation: It is not known whether LONSURF or its metabolites are present in human milk. There are no data to assess the effects of LONSURF or its metabolites on the breast‑fed infant or the effects on milk production. Because of the potential for serious adverse reactions in breast‑fed infants, advise women not to breastfeed during treatment with LONSURF and for 1 day following the final dose.Male Contraception: Because of the potential for genotoxicity, advise males with female partners of reproductive potential to use condoms during treatment with LONSURF and for at least 3 months after the final dose.Geriatric Use: Patients 65 years of age or over who received LONSURF had a higher incidence of the following compared to patients younger than 65 years: Grade 3 or 4 neutropenia (46% vs 32%), Grade 3 anemia (22% vs 16%), and Grade 3 or 4 thrombocytopenia (7% vs 4%).Hepatic Impairment: Do not initiate LONSURF in patients with baseline moderate or severe (total bilirubin greater than 1.5 times ULN and any AST) hepatic impairment. Patients with severe hepatic impairment (total bilirubin greater than 3 times ULN and any AST) were not studied. No adjustment to the starting dose of LONSURF is recommended for patients with mild hepatic impairment.Renal Impairment: No adjustment to the starting dosage of LONSURF is recommended in patients with mild or moderate renal impairment (CLcr of 30 to 89 mL/min). Patients with severe renal impairment (CLcr < 30 mL/min) were not studied.ADVERSE REACTIONSMost Common Adverse Drug Reactions in Patients Treated With LONSURF (≥5%): The most common adverse drug reactions in LONSURF‑treated patients vs placebo‑treated patients with mCRC, respectively, were asthenia/fatigue (52% vs 35%), nausea (48% vs 24%), decreased appetite (39% vs 29%), diarrhea (32% vs 12%), vomiting (28% vs 14%), infections (27% vs 16%), abdominal pain (21% vs 18%), pyrexia (19% vs 14%), stomatitis (8% vs 6%), dysgeusia (7% vs 2%), and alopecia (7% vs 1%). In metastatic gastric cancer or gastroesophageal junction (GEJ), the most common adverse drug reactions respectively were, nausea (37% vs 32%), decreased appetite (34% vs 31%), vomiting (25% vs 20%), infections (23% vs 16%) and diarrhea (23% vs 14%).Pulmonary emboli occurred more frequently in LONSURF‑treated patients compared to placebo: (2% vs 0%) in mCRC and (3% vs 2%) in metastatic gastric cancer and GEJ.Interstitial lung disease (0.2%), including fatalities, has been reported in clinical studies and clinical practice settings in Asia.Laboratory Test Abnormalities in Patients Treated With LONSURF: Laboratory test abnormalities in LONSURF‑treated patients vs placebo‑treated patients with mCRC, respectively, were anemia (77% vs 33%), neutropenia (67% vs 1%), and thrombocytopenia (42% vs 8%). In metastatic gastric cancer or GEJ, the test abnormalities, respectively, were neutropenia (66% vs 4%), anemia (63% vs 38%), and thrombocytopenia (34% vs 9%).用药温馨提示:当您服用此药物时,需定期接受医疗专业人士的检查,以便随时针对其药效、副作用等情况进行监测。本网站所包含的信息旨在为患者提供帮助,不能代替医学建议和治疗。
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 复方曲氟尿苷盐酸/替吡嘧啶薄膜片Lonsurf 15mg/6.14mg Filmtabletten
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 复方曲氟尿苷盐酸/替吡嘧啶薄膜片Lonsurf 15mg/6.14mg Filmtabletten