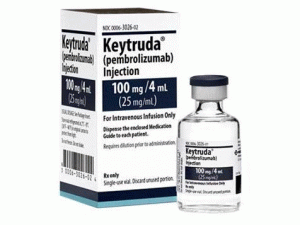
派姆单抗冻干粉注射剂Keytruda 25mg/ml 4nl vial
 药店国别:
产地国家:美国
处方药:是
所属类别: 25毫克/毫升 4毫升/瓶
包装规格: 25毫克/毫升 4毫升/瓶
计价单位:瓶
生产厂家中文参考译名:
生产厂家英文名:MERCK SHARP & DOHME CORP
原产地英文商品名:KEYTRUDA 25MG/ML 4ML SDV 1/EA
原产地英文药品名:pembrolizumab
中文参考商品译名:KEYTRUDA冻干粉注射剂 25毫克/毫升 4毫升/瓶
中文参考药品译名:派姆单抗
曾用名:
药店国别:
产地国家:美国
处方药:是
所属类别: 25毫克/毫升 4毫升/瓶
包装规格: 25毫克/毫升 4毫升/瓶
计价单位:瓶
生产厂家中文参考译名:
生产厂家英文名:MERCK SHARP & DOHME CORP
原产地英文商品名:KEYTRUDA 25MG/ML 4ML SDV 1/EA
原产地英文药品名:pembrolizumab
中文参考商品译名:KEYTRUDA冻干粉注射剂 25毫克/毫升 4毫升/瓶
中文参考药品译名:派姆单抗
曾用名:
简介
美国默克首例PD-1单抗pembrolizumab(商品名 Keytruda®)获FDA批准上市近日,默克PD-1单抗Keytruda®(pembrolizumab)获美国食品药品监督管理局(FDA)批准的首例PD-1单抗。该药适应症为不可切除的或转移性黑色素瘤。 Pembrolizumab是一种新型人源化单抗,通过作用于程序性细胞死亡1(PD - 1的)提升人体免疫力,消灭晚期黑色素瘤。根据临床I期数据显示,24%黑色素瘤患者在接受治疗之后体内的肿瘤大小出现缩小。 批准日期:2014年9月4日; 公司:Merck & Co.,IncKEYTRUDA(派姆单抗[pembrolizumab])注射,用于静脉注射KEYTRUDA(派姆单抗[pembrolizumab])注射,注射用于静脉注射 美国初步批准:2014年 最近的主要变化适应症和用法:05/2017 剂量和管理:07/2017 警告和注意事项:07/2017 作用机制 将PD-1配体,PD-L1和PD-L2与T细胞上发现的PD-1受体的结合抑制T细胞增殖和细胞因子产生。 PD-1配体的上调发生在一些肿瘤中,并且通过该途径的信号传导可以有助于抑制肿瘤的活性T细胞免疫监视。 Pembrolizumab是与PD-1受体结合并阻断其与PD-L1和PD-L2的相互作用的单克隆抗体,释放PD-1途径介导的免疫应答的抑制,包括抗肿瘤免疫应答。 在同基因小鼠肿瘤模型中,阻断PD-1活性导致肿瘤生长减少。 适用范围及用途 KEYTRUDA是一种程序性死亡受体-1(PD-1)阻断抗体,如下所示:黑色素瘤用于治疗不可切除或转移性黑素瘤的患者。非小细胞肺癌(NSCLC)作为用于一线治疗转移性NSCLC患者的单一药物,其肿瘤具有高PD-L1表达[(肿瘤比例评分(TPS)≥50%)],通过FDA批准的测试确定,无EGFR或ALK基因组肿瘤畸变。 作为用于治疗转移性NSCLC患者的单一药剂,其肿瘤表达通过FDA批准的测试确定的PD-L1(TPS≥1%),在含铂化疗后或之后发生疾病进展。患有EGFR或ALK基因组肿瘤畸变的患者在接受KEYTRUDA之前,应在FDA批准的这些畸变治疗中具有疾病进展。与培美曲塞和卡铂组合,作为转移性非鳞癌NSCLC患者的一线治疗。该指征基于肿瘤反应率和无进展生存期的加速批准获得批准。继续批准该指征可能取决于确认试验中临床获益的验证和描述。 头颈部鳞状细胞癌(HNSCC)用于治疗复发性或转移性HNSCC患者,在含铂化疗后,进行疾病进展。该指征根据肿瘤反应率和反应耐久性加快批准。继续批准该指征可能取决于确认试验中临床获益的验证和描述。古典霍奇金淋巴瘤(cHL)用于治疗患有难治性cHL的成人和儿科患者,或者在3次或更多次治疗之后复发。 该指征根据肿瘤反应率和反应耐久性加快批准。继续批准该指征可能取决于确认试验中临床获益的验证和描述。尿路上皮癌用于治疗不符合顺铂化疗的局部晚期或转移性尿路上皮癌患者。该指征根据肿瘤反应率和反应持续时间加快批准。继续批准该指征可能取决于确认试验中临床获益的验证和描述。用于治疗在含铂化疗期间或之后具有疾病进展的局部晚期或转移性尿路上皮癌患者,或在含铂化疗的新辅助或辅助治疗的12个月内治疗。微卫星不稳定性 - 高癌症用于治疗成人和儿科患者 如何提供/存储和处理 注射用KEYTRUDA(冻干粉末):含有一个50毫克单剂量小瓶(NDC 0006-3029-02)的纸箱。在2°C至8°C(36°F至46°F)的冷藏条件下储存小瓶。KEYTRUDA注射液(溶液):含有一个100mg / 4mL(25mg / mL),单剂量小瓶(NDC 0006-3026-02)将小瓶在原装纸箱中的2°C至8°C(36°F至46°F)冷藏保存,以防止光照。 不要冻结 不要动摇 派姆单抗冻干粉注射剂英文版说明书 KEYTRUDA is a prescription medicine used to treat:•a kind of skin cancer called melanoma. It may be used when your melanoma has spread or cannot be removed by surgery (advanced melanoma).•a kind of lung cancer called non–small cell lung cancer (NSCLC). ◦It may be used alone as your first treatment when your lung cancer has spread (advanced NSCLC) and tests positive for “PD-L1” and your tumor does not have an abnormal “EGFR” or “ALK” gene.◦It may also be used alone for advanced NSCLC if you have tried chemotherapy that contains platinum and it did not work or is no longer working and your lung cancer tests positive for “PD-L1” and, if your tumor has an abnormal “EGFR” or “ALK” gene, you have also received an “EGFR” or “ALK” inhibitor medicine that did not work or is no longer working.•a kind of cancer called head and neck squamous cell cancer (HNSCC). It may be used when your HNSCC has returned or spread and you have received chemotherapy that contains platinum and it did not work or is no longer working.•a kind of cancer called classical Hodgkin lymphoma (cHL). KEYTRUDA may be used for cHL in adults and children when you have tried a treatment and it did not work or when your cHL has returned after you received 3 or more types of treatment.•a kind of bladder or urinary tract cancer when it has spread or cannot be removed by surgery (advanced urothelial cancer) and you are not able to receive chemotherapy that contains a medicine called cisplatin, or you have received chemotherapy that contains platinum, and it did not work or is no longer working.•a kind of cancer that is shown by a laboratory test to be a microsatellite instability-high (MSI-H) or a mismatch repair deficient (dMMR) solid tumor. KEYTRUDA may be used in adults and children to treat: ◦cancer that has spread or cannot be removed by surgery (advanced cancer), and◦has progressed following treatment, and you have no satisfactory treatment options, or◦you have colon or rectal cancer, and you have received chemotherapy with fluoropyrimidine, oxaliplatin, and irinotecan but it did not work or is no longer working.It is not known if KEYTRUDA is safe and effective in children with MSI-H cancers of the brain or spinal cord (central nervous system cancers).PD-L1 = programmed death ligand 1;EGFR = epidermal growth factor receptor;ALK = anaplastic lymphoma kinase.Important Safety Information About KEYTRUDA® (pembrolizumab)KEYTRUDA is a medicine that may treat certain cancers by working with your immune system. KEYTRUDA can cause your immune system to attack normal organs and tissues in any area of your body and can affect the way they work. These problems can sometimes become serious or life-threatening and can lead to death.Call or see your doctor right away if you develop any symptoms of the following problems or these symptoms get worse:Lung problems (pneumonitis). Symptoms of pneumonitis may include shortness of breath, chest pain, or new or worse cough.Intestinal problems (colitis) that can lead to tears or holes in your intestine. Signs and symptoms of colitis may include diarrhea or more bowel movements than usual; stools that are black, tarry, sticky, or have blood or mucus; or severe stomach-area (abdomen) pain or tenderness.Liver problems (hepatitis). Signs and symptoms of hepatitis may include yellowing of your skin or the whites of your eyes, nausea or vomiting, pain on the right side of your stomach area (abdomen), dark urine, feeling less hungry than usual, or bleeding or bruising more easily than normal.Hormone gland problems (especially the thyroid, pituitary, adrenal glands, and pancreas). Signs and symptoms that your hormone glands are not working properly may include rapid heartbeat, weight loss or weight gain, increased sweating, feeling more hungry or thirsty, urinating more often than usual, hair loss, feeling cold, constipation, your voice gets deeper, muscle aches, dizziness or fainting, or headaches that will not go away or unusual headache.Kidney problems, including nephritis and kidney failure. Signs of kidney problems may include change in the amount or color of your urine.Skin problems. Signs of skin problems may include rash, itching, blisters, peeling or skin sores, or painful sores or ulcers in your mouth or in your nose, throat, or genital area.Problems in other organs. Signs of these problems may include changes in eyesight, severe or persistent muscle or joint pains, severe muscle weakness, or low red blood cells (anemia), shortness of breath, irregular heartbeat, feeling tired, or chest pain (myocarditis).Infusion (IV) reactions that can sometimes be severe and life-threatening. Signs and symptoms of infusion reactions may include chills or shaking, shortness of breath or wheezing, itching or rash, flushing, dizziness, fever, or feeling like passing out.Rejection of a transplanted organ. People who have had an organ transplant may have an increased risk of organ transplant rejection if they are treated with KEYTRUDA.Complications of stem cell transplantation that uses donor stem cells (allogeneic) after treatment with KEYTRUDA. These complications can be severe and can lead to death. Your doctor will monitor you for signs of complications if you are an allogeneic stem cell transplant recipient.Getting medical treatment right away may help keep these problems from becoming more serious. Your doctor will check you for these problems during treatment with KEYTRUDA. Your doctor may treat you with corticosteroid or hormone replacement medicines. Your doctor may also need to delay or completely stop treatment with KEYTRUDA if you have severe side effects.Before you receive KEYTRUDA, tell your doctor if you have immune system problems such as Crohn’s disease, ulcerative colitis, or lupus; have had an organ transplant; have lung or breathing problems; have liver problems; or have any other medical problems.If you are pregnant or plan to become pregnant, tell your doctor. KEYTRUDA can harm your unborn baby. Females who are able to become pregnant should use an effective method of birth control during treatment and for at least 4 months after the final dose of KEYTRUDA. Tell your doctor right away if you become pregnant during treatment with KEYTRUDA.If you are breastfeeding or plan to breastfeed, tell your doctor. It is not known if KEYTRUDA passes into your breast milk. Do not breastfeed during treatment with KEYTRUDA and for 4 months after your final dose of KEYTRUDA.Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.Common side effects of KEYTRUDA include feeling tired, itching, diarrhea, decreased appetite, rash, fever, cough, shortness of breath, pain in muscles, bones or joints, constipation, and nausea.In children, feeling tired, vomiting and stomach-area (abdominal) pain, and increased levels of liver enzymes and decreased levels of salt (sodium) in the blood are more common than in adults.These are not all the possible side effects of KEYTRUDA.用药温馨提示:当您服用此药物时,需定期接受医疗专业人士的检查,以便随时针对其药效、副作用等情况进行监测。本网站所包含的信息旨在为患者提供帮助,不能代替医学建议和治疗。
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 派姆单抗冻干粉注射剂Keytruda 25mg/ml 4nl vial
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 派姆单抗冻干粉注射剂Keytruda 25mg/ml 4nl vial