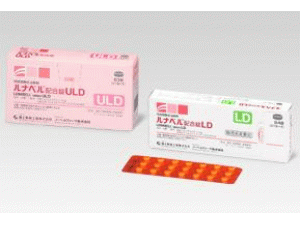
SENSHIO filmcoated tablet(欧司哌米芬薄膜片)说明书
[caption id="attachment_23614" align="alignleft" width="300"] SENSHIO filmcoated tablet(欧司哌米芬薄膜片)[/caption]
药店国别:
产地国家:德国
处 方 药:是
所属类别:60毫克/片 28片/盒
包装规格:60毫克/片 28片/盒
计价单位:盒
生产厂家中文参考译名:盐野义制药
生产厂家英文名:Shionogi
原产地英文商品名:Senshio filmcoated tablet 60MG/Tablets 28TABLETS
原产地英文药品名:ospemifene
中文参考商品译名:Senshio薄膜片 60毫克/片 28片/盒
中文参考药品译名:欧司哌米芬
简介:
部份中文欧司米芬处方资料(仅供参考)
商品名称:Senshio
通用名称:ospemifene
通用名称:欧司米芬
研发阶段:批准上市
原研公司:盐野义制药
作用机制:estrogen agonist/antgonist
适应病症:为治疗中度至重度性交痛,外阴和阴道萎缩症。
用法用量:每日一次,每次60mg(每日1片)
包装规格:60mg*28片
生产厂家:盐野义制药
ospemifene 欧司米芬
欧司米芬由盐野义(shionog)研发,于2013年2月26日首次获美国食品药品管理局(FDA)批准上市,后又于2015年1月15日获欧洲药物管理局(EMA)批准上市,由盐野义在美国和欧洲上市销售。商品名分别为osphena@和senshin@
欧司米芬是一种有组织选择性的雌激素激动剂。其生物学行为是通过与雌激素受体结合介导的。该药适用于治疗中度至重度性交痛,由绝经引起的外阴和阴道萎缩症。
ospemifene为口服薄膜片,每片含有60毫克。推荐剂为每次60mg,与餐同服。
ospemifene(Senshio™) 60mg film-coated tablet
Ospemifene (Senshio) for Vulvar and Vaginal Atrophy
Ospemifene (Senshio, Shionogi Limited) was recommended by a European Medicines Agency (EMA) panel for the treatment of moderate to severe symptomatic vulvar and vaginal atrophy in postmenopausal women who are unable to use local vaginal estrogen therapy, according to an agency news release.
The EMA's Committee for Medicinal Products for Human Use recommended marketing authorization for ospemifene after reviewing quality, safety, and efficacy data.
Ospemifene is a selective estrogen receptor modulator (ATC code G03XC05) that, along with its primary metabolite, binds to estrogen receptors to activate estrogenic pathways in some tissues (agonism) and block estrogenic pathways in other tissues (antagonism). It is available in 60-mg tablets.
Ospemifene improves vaginal pH levels, matures the vaginal epithelium, and relieves troublesome symptoms, including vaginal dryness and dyspareunia (painful intercourse). The most frequently reported adverse effect was hot flushes.
The US Food and Drug Administration approved ospemifene for the treatment of dyspareunia in February 2013 after reviewing data from three clinical trials of 1889 postmenopausal women suffering from vulvar and vaginal atrophy. In those studies, women were randomly assigned to receive either ospemifene or a placebo. After 12 weeks of treatment, women in the first two studies who received ospemifene experienced significantly improved dyspareunia compared with women who received placebo. The third study demonstrated ospemifene's long-term safety in women with dyspareunia.
SENSHIO filmcoated tablet(欧司哌米芬薄膜片)[/caption]
药店国别:
产地国家:德国
处 方 药:是
所属类别:60毫克/片 28片/盒
包装规格:60毫克/片 28片/盒
计价单位:盒
生产厂家中文参考译名:盐野义制药
生产厂家英文名:Shionogi
原产地英文商品名:Senshio filmcoated tablet 60MG/Tablets 28TABLETS
原产地英文药品名:ospemifene
中文参考商品译名:Senshio薄膜片 60毫克/片 28片/盒
中文参考药品译名:欧司哌米芬
简介:
部份中文欧司米芬处方资料(仅供参考)
商品名称:Senshio
通用名称:ospemifene
通用名称:欧司米芬
研发阶段:批准上市
原研公司:盐野义制药
作用机制:estrogen agonist/antgonist
适应病症:为治疗中度至重度性交痛,外阴和阴道萎缩症。
用法用量:每日一次,每次60mg(每日1片)
包装规格:60mg*28片
生产厂家:盐野义制药
ospemifene 欧司米芬
欧司米芬由盐野义(shionog)研发,于2013年2月26日首次获美国食品药品管理局(FDA)批准上市,后又于2015年1月15日获欧洲药物管理局(EMA)批准上市,由盐野义在美国和欧洲上市销售。商品名分别为osphena@和senshin@
欧司米芬是一种有组织选择性的雌激素激动剂。其生物学行为是通过与雌激素受体结合介导的。该药适用于治疗中度至重度性交痛,由绝经引起的外阴和阴道萎缩症。
ospemifene为口服薄膜片,每片含有60毫克。推荐剂为每次60mg,与餐同服。
ospemifene(Senshio™) 60mg film-coated tablet
Ospemifene (Senshio) for Vulvar and Vaginal Atrophy
Ospemifene (Senshio, Shionogi Limited) was recommended by a European Medicines Agency (EMA) panel for the treatment of moderate to severe symptomatic vulvar and vaginal atrophy in postmenopausal women who are unable to use local vaginal estrogen therapy, according to an agency news release.
The EMA's Committee for Medicinal Products for Human Use recommended marketing authorization for ospemifene after reviewing quality, safety, and efficacy data.
Ospemifene is a selective estrogen receptor modulator (ATC code G03XC05) that, along with its primary metabolite, binds to estrogen receptors to activate estrogenic pathways in some tissues (agonism) and block estrogenic pathways in other tissues (antagonism). It is available in 60-mg tablets.
Ospemifene improves vaginal pH levels, matures the vaginal epithelium, and relieves troublesome symptoms, including vaginal dryness and dyspareunia (painful intercourse). The most frequently reported adverse effect was hot flushes.
The US Food and Drug Administration approved ospemifene for the treatment of dyspareunia in February 2013 after reviewing data from three clinical trials of 1889 postmenopausal women suffering from vulvar and vaginal atrophy. In those studies, women were randomly assigned to receive either ospemifene or a placebo. After 12 weeks of treatment, women in the first two studies who received ospemifene experienced significantly improved dyspareunia compared with women who received placebo. The third study demonstrated ospemifene's long-term safety in women with dyspareunia.
 SENSHIO filmcoated tablet(欧司哌米芬薄膜片)[/caption]
药店国别:
产地国家:德国
处 方 药:是
所属类别:60毫克/片 28片/盒
包装规格:60毫克/片 28片/盒
计价单位:盒
生产厂家中文参考译名:盐野义制药
生产厂家英文名:Shionogi
原产地英文商品名:Senshio filmcoated tablet 60MG/Tablets 28TABLETS
原产地英文药品名:ospemifene
中文参考商品译名:Senshio薄膜片 60毫克/片 28片/盒
中文参考药品译名:欧司哌米芬
简介:
部份中文欧司米芬处方资料(仅供参考)
商品名称:Senshio
通用名称:ospemifene
通用名称:欧司米芬
研发阶段:批准上市
原研公司:盐野义制药
作用机制:estrogen agonist/antgonist
适应病症:为治疗中度至重度性交痛,外阴和阴道萎缩症。
用法用量:每日一次,每次60mg(每日1片)
包装规格:60mg*28片
生产厂家:盐野义制药
ospemifene 欧司米芬
欧司米芬由盐野义(shionog)研发,于2013年2月26日首次获美国食品药品管理局(FDA)批准上市,后又于2015年1月15日获欧洲药物管理局(EMA)批准上市,由盐野义在美国和欧洲上市销售。商品名分别为osphena@和senshin@
欧司米芬是一种有组织选择性的雌激素激动剂。其生物学行为是通过与雌激素受体结合介导的。该药适用于治疗中度至重度性交痛,由绝经引起的外阴和阴道萎缩症。
ospemifene为口服薄膜片,每片含有60毫克。推荐剂为每次60mg,与餐同服。
ospemifene(Senshio™) 60mg film-coated tablet
Ospemifene (Senshio) for Vulvar and Vaginal Atrophy
Ospemifene (Senshio, Shionogi Limited) was recommended by a European Medicines Agency (EMA) panel for the treatment of moderate to severe symptomatic vulvar and vaginal atrophy in postmenopausal women who are unable to use local vaginal estrogen therapy, according to an agency news release.
The EMA's Committee for Medicinal Products for Human Use recommended marketing authorization for ospemifene after reviewing quality, safety, and efficacy data.
Ospemifene is a selective estrogen receptor modulator (ATC code G03XC05) that, along with its primary metabolite, binds to estrogen receptors to activate estrogenic pathways in some tissues (agonism) and block estrogenic pathways in other tissues (antagonism). It is available in 60-mg tablets.
Ospemifene improves vaginal pH levels, matures the vaginal epithelium, and relieves troublesome symptoms, including vaginal dryness and dyspareunia (painful intercourse). The most frequently reported adverse effect was hot flushes.
The US Food and Drug Administration approved ospemifene for the treatment of dyspareunia in February 2013 after reviewing data from three clinical trials of 1889 postmenopausal women suffering from vulvar and vaginal atrophy. In those studies, women were randomly assigned to receive either ospemifene or a placebo. After 12 weeks of treatment, women in the first two studies who received ospemifene experienced significantly improved dyspareunia compared with women who received placebo. The third study demonstrated ospemifene's long-term safety in women with dyspareunia.
SENSHIO filmcoated tablet(欧司哌米芬薄膜片)[/caption]
药店国别:
产地国家:德国
处 方 药:是
所属类别:60毫克/片 28片/盒
包装规格:60毫克/片 28片/盒
计价单位:盒
生产厂家中文参考译名:盐野义制药
生产厂家英文名:Shionogi
原产地英文商品名:Senshio filmcoated tablet 60MG/Tablets 28TABLETS
原产地英文药品名:ospemifene
中文参考商品译名:Senshio薄膜片 60毫克/片 28片/盒
中文参考药品译名:欧司哌米芬
简介:
部份中文欧司米芬处方资料(仅供参考)
商品名称:Senshio
通用名称:ospemifene
通用名称:欧司米芬
研发阶段:批准上市
原研公司:盐野义制药
作用机制:estrogen agonist/antgonist
适应病症:为治疗中度至重度性交痛,外阴和阴道萎缩症。
用法用量:每日一次,每次60mg(每日1片)
包装规格:60mg*28片
生产厂家:盐野义制药
ospemifene 欧司米芬
欧司米芬由盐野义(shionog)研发,于2013年2月26日首次获美国食品药品管理局(FDA)批准上市,后又于2015年1月15日获欧洲药物管理局(EMA)批准上市,由盐野义在美国和欧洲上市销售。商品名分别为osphena@和senshin@
欧司米芬是一种有组织选择性的雌激素激动剂。其生物学行为是通过与雌激素受体结合介导的。该药适用于治疗中度至重度性交痛,由绝经引起的外阴和阴道萎缩症。
ospemifene为口服薄膜片,每片含有60毫克。推荐剂为每次60mg,与餐同服。
ospemifene(Senshio™) 60mg film-coated tablet
Ospemifene (Senshio) for Vulvar and Vaginal Atrophy
Ospemifene (Senshio, Shionogi Limited) was recommended by a European Medicines Agency (EMA) panel for the treatment of moderate to severe symptomatic vulvar and vaginal atrophy in postmenopausal women who are unable to use local vaginal estrogen therapy, according to an agency news release.
The EMA's Committee for Medicinal Products for Human Use recommended marketing authorization for ospemifene after reviewing quality, safety, and efficacy data.
Ospemifene is a selective estrogen receptor modulator (ATC code G03XC05) that, along with its primary metabolite, binds to estrogen receptors to activate estrogenic pathways in some tissues (agonism) and block estrogenic pathways in other tissues (antagonism). It is available in 60-mg tablets.
Ospemifene improves vaginal pH levels, matures the vaginal epithelium, and relieves troublesome symptoms, including vaginal dryness and dyspareunia (painful intercourse). The most frequently reported adverse effect was hot flushes.
The US Food and Drug Administration approved ospemifene for the treatment of dyspareunia in February 2013 after reviewing data from three clinical trials of 1889 postmenopausal women suffering from vulvar and vaginal atrophy. In those studies, women were randomly assigned to receive either ospemifene or a placebo. After 12 weeks of treatment, women in the first two studies who received ospemifene experienced significantly improved dyspareunia compared with women who received placebo. The third study demonstrated ospemifene's long-term safety in women with dyspareunia.
用药温馨提示:当您服用此药物时,需定期接受医疗专业人士的检查,以便随时针对其药效、副作用等情况进行监测。本网站所包含的信息旨在为患者提供帮助,不能代替医学建议和治疗。
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » SENSHIO filmcoated tablet(欧司哌米芬薄膜片)说明书
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » SENSHIO filmcoated tablet(欧司哌米芬薄膜片)说明书