帕罗西汀胶囊(paroxetine)说明书
产地国家:美国 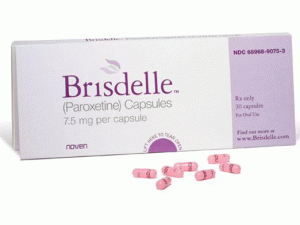 处 方 药:是
所属类别:7.5毫克/胶囊 30胶囊/盒
包装规格:7.5毫克/胶囊 30胶囊/盒
计价单位:盒
生产厂家中文参考译名:NOVEN THERAPEUTICS LLC
生产厂家英文名:NOVEN THERAPEUTICS LLC
原产地英文商品名:BRISDELLE CAP 7.5mg/cap 30caps/bottle
原产地英文药品名:paroxetine mesylate
中文参考商品译名:BRISDELLE胶囊 7.5毫克/胶囊 30胶囊/盒
中文参考药品译名:甲磺酸帕罗西汀
处 方 药:是
所属类别:7.5毫克/胶囊 30胶囊/盒
包装规格:7.5毫克/胶囊 30胶囊/盒
计价单位:盒
生产厂家中文参考译名:NOVEN THERAPEUTICS LLC
生产厂家英文名:NOVEN THERAPEUTICS LLC
原产地英文商品名:BRISDELLE CAP 7.5mg/cap 30caps/bottle
原产地英文药品名:paroxetine mesylate
中文参考商品译名:BRISDELLE胶囊 7.5毫克/胶囊 30胶囊/盒
中文参考药品译名:甲磺酸帕罗西汀
 处 方 药:是
所属类别:7.5毫克/胶囊 30胶囊/盒
包装规格:7.5毫克/胶囊 30胶囊/盒
计价单位:盒
生产厂家中文参考译名:NOVEN THERAPEUTICS LLC
生产厂家英文名:NOVEN THERAPEUTICS LLC
原产地英文商品名:BRISDELLE CAP 7.5mg/cap 30caps/bottle
原产地英文药品名:paroxetine mesylate
中文参考商品译名:BRISDELLE胶囊 7.5毫克/胶囊 30胶囊/盒
中文参考药品译名:甲磺酸帕罗西汀
处 方 药:是
所属类别:7.5毫克/胶囊 30胶囊/盒
包装规格:7.5毫克/胶囊 30胶囊/盒
计价单位:盒
生产厂家中文参考译名:NOVEN THERAPEUTICS LLC
生产厂家英文名:NOVEN THERAPEUTICS LLC
原产地英文商品名:BRISDELLE CAP 7.5mg/cap 30caps/bottle
原产地英文药品名:paroxetine mesylate
中文参考商品译名:BRISDELLE胶囊 7.5毫克/胶囊 30胶囊/盒
中文参考药品译名:甲磺酸帕罗西汀
简介
首个非激素治疗热潮红症的药物Brisdelle(paroxetine mesylate) 获FDA批准上市 美国FDA药物评价和研究中心骨,生殖和泌尿系统部主任Hylton V. Joffe,M.D.,M.M.Sc. 说:“有显著数量妇女患伴绝经潮热和不能或不想用激素治疗”“今天的批准提供妇女第一个被FDA-批准的,非-激素治疗选择帮助缓解绝经中常见的潮热。” BRISDELLE™ (帕罗西汀[paroxetine])胶囊,为口服使用 美国初次批准:2003 作用机制 非临床研究曾显示帕罗西汀是一种SSRI。BRISDELLE不是雌激素,而不知道对治疗血管舒缩症状的作用机制。 适应证和用途 BRISDELLE是一种选择性5-HT摄取抑制剂(SSRI)适用于治疗中度至严重伴绝经血管舒缩症状(VMS) 使用的限制:BRISDELLE不适用于任何精神状况的治疗。 剂量和给药方法 BRISDELLE的推荐剂量是7.5mg每天在睡前1次。 剂型和规格 胶囊:7.5mg 禁忌证 (1)与单胺氧化酶抑制剂(MAOI)同时使用或MAOI使用的14天内使用 (2)与硫利达嗪[thioridazine]使用 (3)与匹莫齐特[pimozide]使用 (4)对BRISDELLE中任何成分超敏性 (5)妊娠 警告和注意事项 (1)自杀:监视自杀或行为不寻常变化 (2)5 -羟色胺综合征:用SSRIs曾报道5 -羟色胺综合征,是潜在地危及生命。中止BRISDELLE和开始支持治疗 (3)他莫昔芬:当与BRISDELLE同时给药时他莫昔芬的疗效可能减低 (4)异常出血:小心患者关于出血伴随BRISDELLE和非甾体抗炎药(NSAIDs),阿司匹林,或影响凝血功能其他药物的同时使用的风险. (5)低钠血症:可能发生伴随抗利尿激素分泌异常综合征(SIADH) (6)骨折:曾报道SSRI治疗和骨折间关联流行病学研究 (7)躁狂/轻躁狂的激活:对躁郁症和监视躁狂/轻躁狂筛选 (8)癫痫发作:有癫痫发作或有可能降低癫痫发作阈值情况史患者谨慎使用 (9)静坐不能:可能发生,大多数很可能在治疗的头几周 (10) 急性闭角型青光眼:在窄角型青光眼患者中可能致急性闭角型青光眼;告诉有窄角型青光眼患者报告视觉症状 (11)认知和运动功能受损:可能致损伤;患者不应操作机械或汽车直至确认BRISDELLE对它们没有不良影响 不良反应 在临床试验中报道的最常见不良反应(≥ 2%)是:头痛,疲乏,和恶心/呕吐 药物相互作用 帕罗西汀是一种强CYP2D6抑制剂。BRISDELLE的共同给药可能改变被CYP2D6代谢的其他药物的浓度。治疗前和期间考虑潜在的药物相互作用。对临床上有意义的药物相互作用清单见完整处方资料. 包装供应/贮存和处置 可得到BRISDELLE为7.5 mg粉红胶囊每粒胶囊用黑色油墨印“NOVEN”和“7.5 mg”。 NDC 68968-9075-3,30粒泡装。 贮存在20°-25°C(68°-77°F);外出允许15°-30°C (59°-86°F)。保护避光和潮气。英文版说明书
Brisdelle(paroxetine mesylate) Pharmacological Class: SSRI. Active Ingredient(s): Paroxetine (as mesylate) 7.5mg; capsules. Company Noven Therapeutics Indication(s): Moderate to severe vasomotor symptoms associated with menopause. Limitation of use: not indicated for treatment of any psychiatric condition. Pharmacology: Brisdelle is an oral selective serotonin reuptake inhibitor (SSRI) and its mechanism of action for the treatment of vasomotor symptoms is unknown. Clinical Trials: The efficacy of Brisdelle was established in two Phase 3 studies in 1174 postmenopausal women with a minimum of 7–8 moderate to severe vasomotor symptoms (VMS) per day at baseline (≥50 per week) for 30 days prior to receiving study drug. Participants received Brisdelle 7.5mg once daily at bedtime. Study 1 was a 12-week, randomized, double-blind, placebo-controlled clinical trial with a total of 606 postmenopausal women. Study 2 was a 24-week, randomized, double-blind, placebo-controlled clinical trial with a total of 568 postmenopausal women. The co-primary efficacy endpoints for both studies were the reduction from baseline in VMS frequency and severity at Weeks 4 and 12. Study 1 results demonstrated a statistically significant reduction from baseline in the frequency of moderate to severe vasomotor symptoms at Week 4 and Week 12 and a statistically significant reduction in the severity of moderate to severe VMS at Week 4 for Brisdelle compared to placebo. Results from Study 2 showed a statistically significant reduction from baseline in the frequency and severity of moderate to severe vasomotor symptoms at Week 4 and Week 12 for Brisdelle compared to placebo. Persistence of benefit at 24 weeks in Study 2 was eva luated with a responder analysis where responders were defined as those patients who achieved ≥50% reduction from baseline in the frequency of moderate to severe VMS at Week 24. The proportion of patients achieving a ≥50% reduction in the frequency of moderate to severe VMS from baseline to Week 24 was 48% in the Brisdelle group and 36% in the placebo group at Week 24. Legal Classification: Rx Adults: 7.5mg once daily at bedtime. Children: Not established. Contraindication(s): During or within 14 days of MAOIs. Concomitant linezolid or IV methylene blue. Concomitant pimozide, thioridazine (may cause QTc prolongation). Pregnancy (Category X). Warnings/Precautions: Increased risk of suicidal thinking and behavior in children and young adults when used to treat depressive or other psychiatric disorders; monitor all patients for clinical worsening or unusual changes; consider discontinuing if occurs. Monitor for serotonin syndrome; discontinue if occurs. Risk of hyponatremia. Volume-depleted. Screen for risk of bipolar disorder prior to initiating therapy; monitor for mania/hypomania. History of seizures or with conditions that lower the seizure threshold; eva luate and consider discontinuing if occurs. Discontinue if akathisia occurs. Narrow angle glaucoma. Elderly. Nursing mothers: not recommended. Interaction(s) See Contraindications. Concomitant other paroxetine-containing products: not recommended. Do not start MAOIs until at least 2 weeks after discontinuing paroxetine. Increased risk of serotonin syndrome with other serotonergic drugs (eg, triptans, tricyclic antidepressants, fentanyl, lithium, tramadol, tryptophan, buspirone, St. John's Wort) or with drugs that impair serotonin metabolism (eg, MAOIs, linezolid, IV methylene blue). Avoid concomitant tamoxifen. May affect, or be affected by, drugs metabolized by CYP2D6, including nortriptyline, amitriptyline, imipramine, desipramine, fluoxetine, phenothiazines, risperidone, atomoxetine, digoxin, theophylline, Class 1C antiarrhythmics (eg, propafenone, flecainide, encainide), quinidine. May potentiate protein-bound drugs (eg, warfarin). Potentiated by cimetidine; monitor. Antagonized by phenobarbital, phenytoin, fosamprenavir/ritonavir; monitor. Increased risk of bleeding with NSAIDs, aspirin, warfarin, or others that affect coagulation. Risk of hyponatremia with diuretics. Adverse Reaction(s) Headache, fatigue, nausea/vomiting; potential for bone fracture. How Supplied: Caps—30 LAST UPDATED: 12/6/2013用药温馨提示:当您服用此药物时,需定期接受医疗专业人士的检查,以便随时针对其药效、副作用等情况进行监测。本网站所包含的信息旨在为患者提供帮助,不能代替医学建议和治疗。
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 帕罗西汀胶囊(paroxetine)说明书
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 帕罗西汀胶囊(paroxetine)说明书