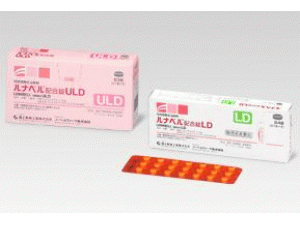
jemina 0.09mg/0.02mg Tablets(左炔诺孕酮/乙炔雌二醇复合片)说明书
[caption id="attachment_22118" align="alignleft" width="300"]
jemina 0.09mg/0.02mg Tablets(左炔诺孕酮/乙炔雌二醇复合片)[/caption] 药店国别: 产地国家:日本 处 方 药:是 所属类别:0.09毫克/0.02毫克/片 84片/盒 包装规格:0.09毫克/0.02毫克/片 84片/盒 计价单位:盒 生产厂家中文参考译名:诺贝尔制药 生产厂家英文名:Nobel Pharma Corporation 原产地英文商品名: jemina(ジェミーナ配合錠)84tabiets/box 原产地英文药品名:Levonorgestrel/Ethinylestradiol 中文参考商品译名:jemina复合片(ジェミーナ配合錠)84片/盒 中文参考药品译名:左炔诺孕酮/乙炔雌二醇 曾用名: 简介: 英文药名:jemina tabiets(Levonorgestrel/Ethinylestradiol) 中文药名:左炔诺孕酮/乙炔雌二醇复合片 日文药名:ジェミーナ配合錠 生产厂家:诺贝尔制药公司 药品介绍 jemina复合片是痛经的治疗剂量方案,也是日本第一个“单相超低剂量雌激素,孕激素的复方制剂” 药物分类名称:雌性激素制剂 批准日期:2018年7月2日 商標名:jemina tabiets 一般名:レボノルゲストレル(Levonorgestrel) 化学名:(-)-13-Ethyl-17-hydroxy-18,19-dinor-17α-pregn-4-en- 20-yn-3-one 分子式:C21H28O2 分子量:312.45 構造式: 性 状: 它是一种白色粉末。 它微溶于四氢呋喃,难溶于乙腈,甲醇,乙醇(99.5)或丙酮,几乎不溶于水。 熔点:232至239℃ 批准条件: 制定药品风险管理计划并适当实施。 药用药理学: 认为该药物通过减轻排卵抑制作用和子宫内膜增殖抑制作用来抑制前列腺素的产生,并减轻由于子宫平滑肌收缩引起的疼痛等。 适应症:痛经 用法与用量: 选择以下之一。 ·每天1片,在固定时间连续21天,然后休息7天。 无论出血是否结束还是继续,从第29天开始下一个周期,以28天为一个周期,重复与上述相同的周期。 ·每天1片,在固定时间连续7天,然后起飞7天。 一个周期超过84天,无论出血是否结束或持续。 包装规格: 0.09毫克/0.02毫克 63片[21片(PTP)×3] 84片[28片(PTP)×3] 制造供应商: Nobel Pharma Corporation 注:以上中文资料不够完整,使用者以原处方资料为准。 Gemina(levonorgestrel/ethynyl estradiol)Domestic first, treatment for dysmenorrhea including levonorgestrel On July 2, 2018, the manufacture and sale of a female hormone preparation containing levonorgestrel/ethinyl estradiol formulation (trade name Geminia combination tablets) was approved. Adaptation is "dysmenorrhea", dosage regimen is "one of the following 28 days 1 cycle (1 day tablet once a day for 21 consecutive days at constant time and 7 days off). 84 One day daily (1 tablet once a day for 77 consecutive days at a fixed time every day for 7 consecutive days, with 7 days rest) In either case the next cycle begins the next day after the end of one cycle regardless of the presence or absence of bleeding Then repeat in the same way ". Dysmenorrhea is a pathological condition such as lower abdominal pain accompanying menstruation during menstruation period, lower back pain, abdominal bloating feeling, nausea, headache, weakness feeling and the like. Dysmenorrhea is characterized by "organic dysmenorrhea" (organic dysmenorrhea) with organic lesions in the pelvis such as endometriosis and "prostaglandin increased in the endometrium" function Severe dysmenorrhea "(primary dysmenorrhea). In the treatment of dysmenorrhea, it is the first choice to use a combination of estrogen (E: follicle hormone) and progestin (P: luteal hormone), and in order to safely use the drug for a long period of time , Drug selection of progestin is important along with low dosage of estrogen. In Japan, low-dose estrogen, estrogen (EE) low-dose estrogen and progestin such as formulation with drospirenone (DRSP) (yards, yards flex) as a progestin, formulation with norethisterone as progestin (lunabel) Dose EP) formulation is used. Geminian combination tablet is Japan's first low-dose EP preparation containing levonorgestrel (LNG) as a progestin in low-dose EE. Three-phase oral contraceptives (eg, Anju, Tricular, etc.) are clinically used as preparations containing the same ingredients. Gemina is the same as the lowest EE content (0.02 mg) among low-dose EP preparations that have indications for dysmenorrhea, and it is characterized by incorporating LNG as a progestin. Research results overseas have confirmed that the EP formulation containing LNG has a lower risk of developing thrombosis than the EP formulation containing DRSP. Moreover, continuous administration of EP preparation is expected to reduce pain by reducing the number of menstrual cycles compared to the 28-day cycle administration, whereas on 28th day administration, breakthrough bleeding is less occurred as compared with continuous administration In some cases, Gemina is an EP formulation that can be selected for any administration method. Efficacy and safety were confirmed on a 28-day cycle administration and continuous administration in a domestic third phase long-term long-term comparison test (placebo-controlled double-blind study) over one year until approval. In case 88.8% of adverse reactions (including clinical laboratory test abnormalities) were observed from the phase III long-term comparison study in Japan. The main things are headaches, nausea, lower abdominal pain, amenorrhea, menorrhagia, illegal uterine bleeding, and an unexpected menstruation, so it is possible that thrombosis may occur as a serious side effect.

jemina 0.09mg/0.02mg Tablets(左炔诺孕酮/乙炔雌二醇复合片)[/caption] 药店国别: 产地国家:日本 处 方 药:是 所属类别:0.09毫克/0.02毫克/片 84片/盒 包装规格:0.09毫克/0.02毫克/片 84片/盒 计价单位:盒 生产厂家中文参考译名:诺贝尔制药 生产厂家英文名:Nobel Pharma Corporation 原产地英文商品名: jemina(ジェミーナ配合錠)84tabiets/box 原产地英文药品名:Levonorgestrel/Ethinylestradiol 中文参考商品译名:jemina复合片(ジェミーナ配合錠)84片/盒 中文参考药品译名:左炔诺孕酮/乙炔雌二醇 曾用名: 简介: 英文药名:jemina tabiets(Levonorgestrel/Ethinylestradiol) 中文药名:左炔诺孕酮/乙炔雌二醇复合片 日文药名:ジェミーナ配合錠 生产厂家:诺贝尔制药公司 药品介绍 jemina复合片是痛经的治疗剂量方案,也是日本第一个“单相超低剂量雌激素,孕激素的复方制剂” 药物分类名称:雌性激素制剂 批准日期:2018年7月2日 商標名:jemina tabiets 一般名:レボノルゲストレル(Levonorgestrel) 化学名:(-)-13-Ethyl-17-hydroxy-18,19-dinor-17α-pregn-4-en- 20-yn-3-one 分子式:C21H28O2 分子量:312.45 構造式: 性 状: 它是一种白色粉末。 它微溶于四氢呋喃,难溶于乙腈,甲醇,乙醇(99.5)或丙酮,几乎不溶于水。 熔点:232至239℃ 批准条件: 制定药品风险管理计划并适当实施。 药用药理学: 认为该药物通过减轻排卵抑制作用和子宫内膜增殖抑制作用来抑制前列腺素的产生,并减轻由于子宫平滑肌收缩引起的疼痛等。 适应症:痛经 用法与用量: 选择以下之一。 ·每天1片,在固定时间连续21天,然后休息7天。 无论出血是否结束还是继续,从第29天开始下一个周期,以28天为一个周期,重复与上述相同的周期。 ·每天1片,在固定时间连续7天,然后起飞7天。 一个周期超过84天,无论出血是否结束或持续。 包装规格: 0.09毫克/0.02毫克 63片[21片(PTP)×3] 84片[28片(PTP)×3] 制造供应商: Nobel Pharma Corporation 注:以上中文资料不够完整,使用者以原处方资料为准。 Gemina(levonorgestrel/ethynyl estradiol)Domestic first, treatment for dysmenorrhea including levonorgestrel On July 2, 2018, the manufacture and sale of a female hormone preparation containing levonorgestrel/ethinyl estradiol formulation (trade name Geminia combination tablets) was approved. Adaptation is "dysmenorrhea", dosage regimen is "one of the following 28 days 1 cycle (1 day tablet once a day for 21 consecutive days at constant time and 7 days off). 84 One day daily (1 tablet once a day for 77 consecutive days at a fixed time every day for 7 consecutive days, with 7 days rest) In either case the next cycle begins the next day after the end of one cycle regardless of the presence or absence of bleeding Then repeat in the same way ". Dysmenorrhea is a pathological condition such as lower abdominal pain accompanying menstruation during menstruation period, lower back pain, abdominal bloating feeling, nausea, headache, weakness feeling and the like. Dysmenorrhea is characterized by "organic dysmenorrhea" (organic dysmenorrhea) with organic lesions in the pelvis such as endometriosis and "prostaglandin increased in the endometrium" function Severe dysmenorrhea "(primary dysmenorrhea). In the treatment of dysmenorrhea, it is the first choice to use a combination of estrogen (E: follicle hormone) and progestin (P: luteal hormone), and in order to safely use the drug for a long period of time , Drug selection of progestin is important along with low dosage of estrogen. In Japan, low-dose estrogen, estrogen (EE) low-dose estrogen and progestin such as formulation with drospirenone (DRSP) (yards, yards flex) as a progestin, formulation with norethisterone as progestin (lunabel) Dose EP) formulation is used. Geminian combination tablet is Japan's first low-dose EP preparation containing levonorgestrel (LNG) as a progestin in low-dose EE. Three-phase oral contraceptives (eg, Anju, Tricular, etc.) are clinically used as preparations containing the same ingredients. Gemina is the same as the lowest EE content (0.02 mg) among low-dose EP preparations that have indications for dysmenorrhea, and it is characterized by incorporating LNG as a progestin. Research results overseas have confirmed that the EP formulation containing LNG has a lower risk of developing thrombosis than the EP formulation containing DRSP. Moreover, continuous administration of EP preparation is expected to reduce pain by reducing the number of menstrual cycles compared to the 28-day cycle administration, whereas on 28th day administration, breakthrough bleeding is less occurred as compared with continuous administration In some cases, Gemina is an EP formulation that can be selected for any administration method. Efficacy and safety were confirmed on a 28-day cycle administration and continuous administration in a domestic third phase long-term long-term comparison test (placebo-controlled double-blind study) over one year until approval. In case 88.8% of adverse reactions (including clinical laboratory test abnormalities) were observed from the phase III long-term comparison study in Japan. The main things are headaches, nausea, lower abdominal pain, amenorrhea, menorrhagia, illegal uterine bleeding, and an unexpected menstruation, so it is possible that thrombosis may occur as a serious side effect.
用药温馨提示:当您服用此药物时,需定期接受医疗专业人士的检查,以便随时针对其药效、副作用等情况进行监测。本网站所包含的信息旨在为患者提供帮助,不能代替医学建议和治疗。
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » jemina 0.09mg/0.02mg Tablets(左炔诺孕酮/乙炔雌二醇复合片)说明书
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » jemina 0.09mg/0.02mg Tablets(左炔诺孕酮/乙炔雌二醇复合片)说明书