咪康唑_ORAVI_咪康唑口腔用含片miconazole说明书
[caption id="attachment_32142" align="alignleft" width="300"]
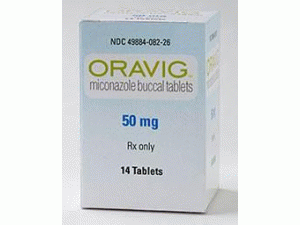
ORAVI Mucoadhesive Tablets 50mg(miconazole 咪康唑口腔用含片)[/caption] 药店国别: 产地国家:日本 处 方 药:是 所属类别:50毫克/片 14片/瓶 包装规格:50毫克/片 14片/瓶 计价单位:瓶 生产厂家中文参考译名:Soken Co.Ltd. 生产厂家英文名:Soken Co.Ltd. 原产地英文商品名:ORAVI50mg/Tablets 14Tablets/bottle 原产地英文药品名:miconazole 中文参考商品译名:ORAVI含片50毫克/片 14片/瓶 中文参考药品译名:咪康唑 简介: 部份中文咪康唑处方资料(仅供参考) 英文名:miconazole 商标名:ORAVI Mucoadhesive Tablets 50mg 中文名:咪康唑 生产商:Soken Co.Ltd. 药效分类:口腔粘膜附着型口腔溃疡治疗药物 批准日期:2006年10月 商标名:ORAVI Mucoadhesive Tablets 50mg 一般名:miconazole 化学名:1-[(2RS)-2-(2,4-dichlorobenzyloxy)-2-(2,4-dichlorophenyl)ethyl] -1H-imidazole 分子式:C18H14Cl4N2O 分子量:416.13 性 状: 是白色~微黄白色的结晶性粉末。 本品容易溶于甲醇、乙醇(95)或醋酸(100),更容易溶于吉埃尔乙醇,几乎不溶于水里。 熔点:84~87℃ 药效药理: 1.对阿齐达的作用 最小发育阻止浓度 对于口腔咽头干达症患者的candida下属临床分离株的最小发育阻止浓度(mic50/ mic90)。 2.作用机制 髓磷脂作用于酪氨酸p450依赖性14α。6)同位素对于高浓度具有可以引起细胞坏死性变化的杀菌作用。 适应症:口腔溃疡 用法与用量:通常,成人每天有一次上颚牙密窝(50毫克)。 临床成绩: 国内临床成绩: 以口腔咽咽干头症患者为对象,以检查使用1次1次,每天使用14天1次的复用性和安全性为目的,进行了把米克纳索尔剂作为对照的随机化非盲检查并行组比较测试。对于开始使用试验药物的第15天的病变得分和症状分数为基础的临床效果,治愈率为本药剂群46.8%(29/62例子),橘子油凝胶剂群47.5%(29/61例子)。 包装:14粒:[含干剂的塑料瓶子] 制造商:Soken Co.Ltd. Approval of domestic manufacturing sales approval of oral oropharyngeal candidiasis drug "orabi tablet oral cavity" SOH group, a 100% subsidiary of soken group, has received approval for manufacturing sales of "orabi tablets oral 50 mg." SOH group, a 100 percent subsidiary of soken group, has received approval for the sale of the product of "orabi tablets oral 50 mg" (hereinafter, "orrabi") on September 21 in Japan. "Orrabi" (development code so-1105) is a new drug type medicinal product of the daily mucosa miconazole which is an antifungicide of the oral mucosa attachment type once administered once a day to treat oropharyngeal candidiasis which contracting the immune function in patients who lowered the immune function. The technology used for the development of "orabi" was called lauriad, and was developed to provide high concentration of active components directly to the local site. Regarding the domestic sale of "orrabi", we have granted exclusive sales right to the group company of Fuji Film Co., Ltd. The company has received 200 million yen as milestone at the time of the approval acquisition, and after the sales start, it has the right to receive milestone separately at the time of the loyalty and a predetermined decision of the sales target. "Orrabi" was developed by the French pharmaceutical company bioalliance Pharma, and was first approved in France in October 2006. Since then, it has been sold in two European and US registered trademarks of oravir /loramycrr. In the end of May 2011, we have obtained the exclusive development right of orobi from bioalliance pharma. The efficacy and safety of "orabi" were confirmed using the data of the randomized phase III clinical trial which carried out the clinical clinical trial and the addition of mikonazole containing pharmaceutical as a control drug in Japan, and the medicine in Japan Approval of product manufacturing sales approval was obtained. Tadayoshi Yasui, President of the president, said: I am glad that Obama will be able to obtain the sales approval in Japan and the way to start sales in the coming months. We would like to express our gratitude to the clinical researchers, hospital staff and patients to participate in our clinical development program and to make our approval possible. The sales partner of this drug is scheduled for the Fuji film Toyama Chemical Co., Ltd., established on October 1, and is convinced that "orrabi" will be a useful treatment for the patient. " In addition, we expect that the impact of this issue on our earnings forecast is slight. Reference Outline of orabi r tablet oral 50mg Approval date Production supplier Co.Ltd. Orrabi r tablets 50mg oral cavity Containing 50 mg of niconazole Oral oropharyngeal candidiasis Oral oropharyngeal candidiasis is a fungal infection mainly caused by Candida albicans (Candida albicans). It is often seen in patients suffering from immunodeficiency such as HIV and malignant tumors, and there are types such as pseudophakic candidiasis and erythema (atrophic) candidiasis. Symptoms include tongue pain, caution, taste abnormality, swallowing difficulty, and white mossy, erythema lesion, and stomatitis. With aging and medical technology advances, candidiasis is increasing.

ORAVI Mucoadhesive Tablets 50mg(miconazole 咪康唑口腔用含片)[/caption] 药店国别: 产地国家:日本 处 方 药:是 所属类别:50毫克/片 14片/瓶 包装规格:50毫克/片 14片/瓶 计价单位:瓶 生产厂家中文参考译名:Soken Co.Ltd. 生产厂家英文名:Soken Co.Ltd. 原产地英文商品名:ORAVI50mg/Tablets 14Tablets/bottle 原产地英文药品名:miconazole 中文参考商品译名:ORAVI含片50毫克/片 14片/瓶 中文参考药品译名:咪康唑 简介: 部份中文咪康唑处方资料(仅供参考) 英文名:miconazole 商标名:ORAVI Mucoadhesive Tablets 50mg 中文名:咪康唑 生产商:Soken Co.Ltd. 药效分类:口腔粘膜附着型口腔溃疡治疗药物 批准日期:2006年10月 商标名:ORAVI Mucoadhesive Tablets 50mg 一般名:miconazole 化学名:1-[(2RS)-2-(2,4-dichlorobenzyloxy)-2-(2,4-dichlorophenyl)ethyl] -1H-imidazole 分子式:C18H14Cl4N2O 分子量:416.13 性 状: 是白色~微黄白色的结晶性粉末。 本品容易溶于甲醇、乙醇(95)或醋酸(100),更容易溶于吉埃尔乙醇,几乎不溶于水里。 熔点:84~87℃ 药效药理: 1.对阿齐达的作用 最小发育阻止浓度 对于口腔咽头干达症患者的candida下属临床分离株的最小发育阻止浓度(mic50/ mic90)。 2.作用机制 髓磷脂作用于酪氨酸p450依赖性14α。6)同位素对于高浓度具有可以引起细胞坏死性变化的杀菌作用。 适应症:口腔溃疡 用法与用量:通常,成人每天有一次上颚牙密窝(50毫克)。 临床成绩: 国内临床成绩: 以口腔咽咽干头症患者为对象,以检查使用1次1次,每天使用14天1次的复用性和安全性为目的,进行了把米克纳索尔剂作为对照的随机化非盲检查并行组比较测试。对于开始使用试验药物的第15天的病变得分和症状分数为基础的临床效果,治愈率为本药剂群46.8%(29/62例子),橘子油凝胶剂群47.5%(29/61例子)。 包装:14粒:[含干剂的塑料瓶子] 制造商:Soken Co.Ltd. Approval of domestic manufacturing sales approval of oral oropharyngeal candidiasis drug "orabi tablet oral cavity" SOH group, a 100% subsidiary of soken group, has received approval for manufacturing sales of "orabi tablets oral 50 mg." SOH group, a 100 percent subsidiary of soken group, has received approval for the sale of the product of "orabi tablets oral 50 mg" (hereinafter, "orrabi") on September 21 in Japan. "Orrabi" (development code so-1105) is a new drug type medicinal product of the daily mucosa miconazole which is an antifungicide of the oral mucosa attachment type once administered once a day to treat oropharyngeal candidiasis which contracting the immune function in patients who lowered the immune function. The technology used for the development of "orabi" was called lauriad, and was developed to provide high concentration of active components directly to the local site. Regarding the domestic sale of "orrabi", we have granted exclusive sales right to the group company of Fuji Film Co., Ltd. The company has received 200 million yen as milestone at the time of the approval acquisition, and after the sales start, it has the right to receive milestone separately at the time of the loyalty and a predetermined decision of the sales target. "Orrabi" was developed by the French pharmaceutical company bioalliance Pharma, and was first approved in France in October 2006. Since then, it has been sold in two European and US registered trademarks of oravir /loramycrr. In the end of May 2011, we have obtained the exclusive development right of orobi from bioalliance pharma. The efficacy and safety of "orabi" were confirmed using the data of the randomized phase III clinical trial which carried out the clinical clinical trial and the addition of mikonazole containing pharmaceutical as a control drug in Japan, and the medicine in Japan Approval of product manufacturing sales approval was obtained. Tadayoshi Yasui, President of the president, said: I am glad that Obama will be able to obtain the sales approval in Japan and the way to start sales in the coming months. We would like to express our gratitude to the clinical researchers, hospital staff and patients to participate in our clinical development program and to make our approval possible. The sales partner of this drug is scheduled for the Fuji film Toyama Chemical Co., Ltd., established on October 1, and is convinced that "orrabi" will be a useful treatment for the patient. " In addition, we expect that the impact of this issue on our earnings forecast is slight. Reference Outline of orabi r tablet oral 50mg Approval date Production supplier Co.Ltd. Orrabi r tablets 50mg oral cavity Containing 50 mg of niconazole Oral oropharyngeal candidiasis Oral oropharyngeal candidiasis is a fungal infection mainly caused by Candida albicans (Candida albicans). It is often seen in patients suffering from immunodeficiency such as HIV and malignant tumors, and there are types such as pseudophakic candidiasis and erythema (atrophic) candidiasis. Symptoms include tongue pain, caution, taste abnormality, swallowing difficulty, and white mossy, erythema lesion, and stomatitis. With aging and medical technology advances, candidiasis is increasing.
用药温馨提示:当您服用此药物时,需定期接受医疗专业人士的检查,以便随时针对其药效、副作用等情况进行监测。本网站所包含的信息旨在为患者提供帮助,不能代替医学建议和治疗。
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 咪康唑_ORAVI_咪康唑口腔用含片miconazole说明书
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 咪康唑_ORAVI_咪康唑口腔用含片miconazole说明书