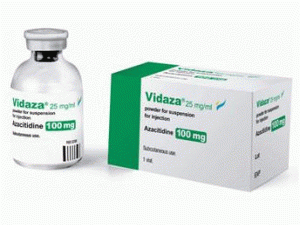
阿扎胞苷,阿扎胞苷冻干粉注射剂(VIDAZA 100MG SDV PWD)
 药店国别:无
产地国家:美国
处方药:是
所属类别: 100毫克/瓶 1瓶/盒
包装规格: 100毫克/瓶 1瓶/盒
计价单位:瓶
生产厂家中文参考译名:无
生产厂家英文名:CELGENE
原产地英文商品名:VIDAZA 100MG SDV PWD 1/EA
原产地英文药品名:AZACITIDINE
中文参考商品译名:维达扎注射剂 100毫克/瓶 1瓶
中文参考药品译名:阿扎胞苷
曾用名:无
药店国别:无
产地国家:美国
处方药:是
所属类别: 100毫克/瓶 1瓶/盒
包装规格: 100毫克/瓶 1瓶/盒
计价单位:瓶
生产厂家中文参考译名:无
生产厂家英文名:CELGENE
原产地英文商品名:VIDAZA 100MG SDV PWD 1/EA
原产地英文药品名:AZACITIDINE
中文参考商品译名:维达扎注射剂 100毫克/瓶 1瓶
中文参考药品译名:阿扎胞苷
曾用名:无
简介
部份中文阿扎胞苷处方资料(仅供参考) 英文名称:Azacitidine 商品名称:Vidaza 中文药名:阿扎胞苷 英文别名:5-Azacytidine、Ladakamycin 开发与上市厂商:5-azacitidine由Pharmion公司开发,于2004年7月在美国首次上市。 作用机制 嘧啶类抗代谢药,干扰核苷酸的合成,以假嘧啶形式掺入DNA和RNA中,并与之结合。 适应症:急性非淋巴细胞性白血病。用于乳腺癌、肠癌、黑色素瘤等有一定疗效。 常用剂量:100mg/m2,静脉推注,每8小时一次,连用5天,200mg/(m2·d)静脉持续滴注,连用5天。 注意事项 由于药物的稳定性差,因此应该在用药前配药,配药后立即使用,配药后8小时未用则应丢弃。静脉滴注应该用新鲜林格液配制,每8小时配制一次。 毒性反应 (1)骨髓抑制和其他血液学反应:所有的病人都会出现严重的骨髓抑制和其他血液学反应,表现为白细胞于第12~14天降至最低,偶见抑制持续超过几周。 (2)恶心呕吐和其他胃肠道反应:常见。静脉持续滴注可减轻恶心呕吐和其他胃肠道反应反应。 (3)皮肤粘膜反应:粘膜炎及皮肤红疹偶见 (4)其他反应:1)腹泻:常见。2)神经系统反应:肌肉疼痛、虚弱、嗜睡及昏迷,少见。3)肝毒性:罕见,但可能严重。4)暂时性发热:偶见。 Vidaza(azacitidine)注射液为第一个治疗骨髓增生异常综合症。英文版说明
VIDAZA is indicated for treatment of patients with the following French-American-British (FAB) myelodysplastic syndrome subtypes: refractory anemia (RA) or refractory anemia with ringed sideroblasts (RARS) (if accompanied by neutropenia or thrombocytopenia or requiring transfusions), refractory anemia with excess blasts (RAEB), refractory anemia with excess blasts in transformation (RAEB-T), and chronic myelomonocytic leukemia (CMMoL).IMPORTANT SAFETY INFORMATION•VIDAZA is contraindicated in patients with a known hypersensitivity to azacitidine or mannitol and in patients with advanced malignant hepatic tumors.•In Studies 1 and 2, the most commonly occurring adverse reactions by SC route were nausea (70.5%), anemia (69.5%), thrombocytopenia (65.5%), vomiting (54.1%), pyrexia (51.8%), leukopenia (48.2%), diarrhea (36.4%), injection site erythema (35.0%), constipation (33.6%), neutropenia (32.3%), and ecchymosis (30.5%). Other adverse reactions included dizziness (18.6%), chest pain (16.4%), febrile neutropenia (16.4%), myalgia (15.9%), injection site reaction (13.6%), and malaise (10.9%). In Study 3, the most common adverse reactions by IV route also included petechiae (45.8%), weakness (35.4%), rigors (35.4%), and hypokalemia (31.3%).•In Study 4, the most commonly occurring adverse reactions were thrombocytopenia (69.7%), neutropenia (65.7%), anemia (51.4%), constipation (50.3%), nausea (48.0%), injection site erythema (42.9%), and pyrexia (30.3%). The most commonly occurring Grade 3/4 adverse reactions were neutropenia (61.1%), thrombocytopenia (58.3%), leukopenia (14.9%), anemia (13.7%) and febrile neutropenia (12.6%).•Because treatment with VIDAZA is associated with anemia, neutropenia and thrombocytopenia, complete blood counts should be performed as needed to monitor response and toxicity, but at a minimum, prior to each dosing cycle.•Because azacitidine is potentially hepatotoxic in patients with severe preexisting hepatic impairment, caution is needed in patients with liver disease. In addition, azacitidine and its metabolites are substantially excreted by the kidneys and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, it may be useful to monitor renal function.•VIDAZA may cause fetal harm when administered to a pregnant woman. Women of childbearing potential should be apprised of the potential hazard to the fetus. Men should be advised not to father a child while receiving VIDAZA.•Nursing mothers should be advised to discontinue nursing or the drug, taking into consideration the importance of the drug to the mother.Please see full Prescribing Information.VIDAZA® is a registered trademark of and Celgene Patient Support™ is a trademark of Celgene Corporation.©2010 Celgene Corporation. All rights reserved.用药温馨提示:当您服用此药物时,需定期接受医疗专业人士的检查,以便随时针对其药效、副作用等情况进行监测。本网站所包含的信息旨在为患者提供帮助,不能代替医学建议和治疗。
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 阿扎胞苷,阿扎胞苷冻干粉注射剂(VIDAZA 100MG SDV PWD)
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 阿扎胞苷,阿扎胞苷冻干粉注射剂(VIDAZA 100MG SDV PWD)