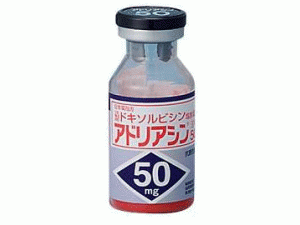
盐酸多柔比星注射剂(ADRIACIN Injection 50mg)
 药店国别:无
产地国家:日本
处方药:是
所属类别: 50毫克/毫升 1瓶
包装规格: 50毫克/毫升 1瓶
计价单位:盒
生产厂家中文参考译名:无
生产厂家英文名:Kyowa Hakko Kirin Co., Ltd.
原产地英文商品名:ADRIACIN Injection(アドリアシン注用)50mg/ml/vials 1vials
原产地英文药品名:Doxorubicin Hydrochloride
中文参考商品译名:ADRIACIN注射剂(アドリアシン注用)50毫克/毫升 1瓶
中文参考药品译名:盐酸多柔比星
曾用名:无
药店国别:无
产地国家:日本
处方药:是
所属类别: 50毫克/毫升 1瓶
包装规格: 50毫克/毫升 1瓶
计价单位:盒
生产厂家中文参考译名:无
生产厂家英文名:Kyowa Hakko Kirin Co., Ltd.
原产地英文商品名:ADRIACIN Injection(アドリアシン注用)50mg/ml/vials 1vials
原产地英文药品名:Doxorubicin Hydrochloride
中文参考商品译名:ADRIACIN注射剂(アドリアシン注用)50毫克/毫升 1瓶
中文参考药品译名:盐酸多柔比星
曾用名:无
简介:
英文药名:ADRIACIN Injection(Doxorubicin Hydrochloride) 中文药名:盐酸多柔比星注射剂 生产厂家:协和发酵麒麟治疗类别名称抗肿瘤药 批准日期:2006年6月 欧文商标名ADRIACIN Injection一般名ドキソルビシン塩酸塩 Doxorubicin Hydrochloride慣用名アドリアマイシン Adriamycin化学名(2S,4S)-4-(3-Amino-2,3,6-trideoxy-α-L-lyxo-hexopyranosyloxy)-2,5,12-trihydroxy-2-hydroxyacetyl-7-methoxy-1,2,3,4-tetrahydrotetracene-6,11-dione monohydrochloride分子式C27H29NO11・HCl=579.98化学構造式性状是红橙色结晶粉末。可溶性略少溶于水,略溶于甲醇,极微溶于乙醇(99.5),并在乙腈中几乎不溶。分配系数logP'OCT=1.4[ 测量方法:摇瓶法正辛醇/pH7.4缓冲溶液]药效药理
1.抗肿瘤具有广泛的抗癌谱移植癌症,艾氏腹水癌,S180肉瘤,肝癌MH-134,淋巴瘤6C3HED·OG,L-1210,显示了对吉田肉瘤等较强的抗肿瘤作用.另外,该药物表现出抗吉田肉瘤成为耐其它药物如丝裂霉素C,5-FU的抗肿瘤效果。 2. 作用机制通过形成DNA和复合在肿瘤细胞中,DNA聚合酶反应,抑制RNA聚合酶反应,DNA,通过抑制RNA的合成显示出抗肿瘤作用。 适应症◇盐酸阿霉素通常治疗以下各种疾病的主观和TaSatoshi症状缓解恶性淋巴瘤,肺癌,胃肠癌(胃癌,胆囊癌,胆管癌,胰腺癌,肝癌,结肠癌,直肠癌,等),乳癌,膀胱癌,骨肉瘤联合治疗与其他抗肿瘤剂以下恶性肿瘤乳腺癌(术前在手术可以是一个例子或术后化疗,),子宫内膜癌(术后化疗,转移和化疗过程中复发),恶性骨和软肿瘤,恶性骨肿瘤,多发性骨髓瘤,儿科恶性肿瘤实体瘤(尤因氏肉瘤家族肿瘤,横纹肌肉瘤,神经母细胞瘤,视网膜母细胞瘤,肝母细胞瘤,肾母细胞瘤肿瘤,等) 用法与用量:◇(胃癌,胆囊癌,胆管癌,胰腺癌,肝癌,结肠癌,直肠癌等),乳腺癌,骨肉瘤的用药情况如下: 1)每日剂量,溶解在10毫克(0.2mg的/公斤)日本药典注射用水或日本药典盐水作为盐酸阿霉素,一次,连续4-6天后静脉内单次给药,每天7-10天,然后停药。 2)每日剂量,溶解在20毫克(为0.4mg/kg)的日本药典注射用水或日本药典盐水作为盐酸阿霉素,单次给药静脉内一次2-3天后一天,7-10天,然后停药。 3)每日剂量,溶解成盐酸阿霉素20〜30毫克(0.4〜0.6mg的/千克)在日本药典注射用水或日本药典盐水,一天一次,一个在每日3天静脉内效力拍摄给药后,然后停药18天。 4)总剂量500毫克作为盐酸阿霉素/米2和(体表面积)以下。◇在恶性淋巴瘤用药的情况下 5)上述1)根据〜3)。 6)在与其它抗癌剂,剂量和标准盐酸阿霉素的方法组合如下。(1)每日一次作为盐酸阿霉素25〜50毫克/米体表面积的2给药以至少2周或更长时间的间隔静脉内施用重复。(2)每日1次40毫克/米2(体表面积),8天的/米为30mg@2(体表面积)通过静脉内施用,随后停药20天。该方法为一个疗程,重复给药。在给药时溶解在日本药典注射用水或日本药典盐水洗涤,用输液稀释根据需要。此外,这取决于患者的状况年龄,伴随药物,体重损失为适当。盐酸阿霉素的总剂量不超过500毫克/米2(体表面积)。◇用于联合治疗的抗乳腺癌其它抗癌剂(术前在可操作的例子或术后化疗) 7)用日本药典注射用水环磷酰胺水合物,用量和标准盐酸阿霉素的给药方法,每日剂量1,60毫克作为盐酸阿霉素/平方米(体表面积)的组合或溶解在日本药典生理盐水,每日一次静脉内给药后,随后停药20天。此外,根据患者的年龄,症状来用药。盐酸阿霉素的总剂量不超过500毫克/米2(体表面积)。◇对于组合疗法与针对子宫内膜癌等抗肿瘤剂(术后化疗,化疗期间转移或复发) 8)在用日本药典的水顺铂,多柔比星的标准盐酸的剂量和施用方法,每日剂量1,60毫克作为盐酸阿霉素/平方米(体表面积)用于注射或日本药典盐水组合溶解于,给药一天一次静脉内,然后重复冲洗和每三周。此外,根据患者的年龄,症状用药。盐酸阿霉素的总剂量不超过500毫克/米2(体表面积)。◇在联合疗法中与恶性骨和软肿瘤其他抗肿瘤剂的情况下 9)与异环磷酰胺,用于注射或日本药典生理的日本药典水剂量和标准盐酸阿霉素的给药方法,每日剂量1,20〜30毫克作为盐酸阿霉素/平方米(体表面积)的组合溶解在盐水中,将其静脉内连续每天给予一次持续3天,然后休息和重复每3-4周。应当指出的是,根据患者的年龄,症状来减肥。盐酸阿霉素的总剂量不超过500毫克/米2(体表面积)。在该药物单独3),根据4)。◇在联合疗法中与恶性骨肿瘤其他抗肿瘤剂的情况下 10)在用日本药典的水顺铂,多柔比星的标准盐酸的剂量和使用方法,每日剂量1,20毫克作为盐酸阿霉素/平方米(体表面积)用于注射或日本药典盐水组合溶解于,它分配静脉或静脉滴注连续每日一次,连续3天,然后停药3周。这是一个很酷的,重复的管理。此外,体重减轻适当的疾病和症状。盐酸阿霉素的总剂量不超过500毫克/米2(体表面积)。◇对于组合疗法与其他抗癌剂为多发性骨髓瘤 11)硫酸长春新碱,在日本药典注射地塞米松磷酸酯钠,用量和标准盐酸阿霉素的给药方法,9毫克,为每日剂量盐酸阿霉素/平方米(体表面积)的组合溶解于水或日本药典盐水,并根据需要在输注稀释24小时连续静脉输注。为此,它会在连续四天。然后洗出,重复每3〜4周和1冷的方法。此外,根据患者的年龄,症状来减肥。盐酸阿霉素的总剂量不超过500毫克/米2(体表面积)。◇儿科恶性实体肿瘤(尤因氏肉瘤家族肿瘤,横纹肌肉瘤,神经母细胞瘤,视网膜母细胞瘤,肝母细胞瘤,肾母细胞瘤肿瘤,等)在组合疗法中与其他抗肿瘤剂的情况下 12)在与其它抗癌剂,剂量和标准盐酸阿霉素的方法组合如下。(1) 1天20〜40毫克/平方米(体表面积)和24小时连续输注1次20〜80毫克/米给药经24至96小时体表面积,在重复的情况下在至少三个周的间隔施用。每日剂量和最大40毫克/米2(体表面积)。(2) 静脉注射或静脉滴注的每天20〜40毫克/平方米(体表面积)1场20〜80毫克/米2施用(体表面积)在壳体的至少三个周的间隔施用的重复。每日剂量和最大40毫克/米2(体表面积)。在给药时溶解在日本药典注射用水或日本药典盐水洗涤,用输液稀释根据需要。此外,这取决于患者的状况年龄,伴随药物,体重损失为适当。盐酸阿霉素的总剂量不超过500毫克/米2(体表面积)。◇在膀胱肿瘤中的情况下 13)每日剂量,溶解于1〜2毫克/mL的如在日本药典盐水盐酸阿霉素毫克30〜60 20〜40毫升,每天1次,每天一次或每周2至3次注射到膀胱内腔。此外,适当增加或者根据年龄和症状减少。(盐酸阿霉素的膀胱内注入法)和尿与导尿管,充分地从导管膀胱内排空后,盐酸阿霉素30〜60毫克1〜在日本药典盐水20〜40毫升2毫克的效价/将其溶解,这将成为毫升注入膀胱内腔,1-2小时至膀胱。◇M-VAC治疗:尿路上皮癌用法与用量氨甲蝶呤,与硫酸长春碱,顺铂组合,通常由盐酸阿霉素日本药典注射用水或日本药典盐水,成人一次30毫克/米溶解@体表面积的2静脉内注射。应当指出的是,根据患者的年龄,症状用药。 标准剂量和施用方法:甲氨蝶呤30毫克/米2,第1天,硫酸长春花碱第2天3毫克/米2,盐酸阿霉素30毫克/米2,顺铂70毫克给药后/米2静脉注射。第15天及第22天注射甲氨蝶呤30毫克/米2和硫酸长春碱3毫克/米2静脉内给药。这是每4周重复为一个疗程,但盐酸阿霉素和500mg/米2或更小的总剂量。 包装规格:注射剂10毫克:10瓶50毫克:1瓶英文版说明:
https://www.info.pmda.go.jp/go/pack/4235402D1030_1_12/Anticancer antibiotics (anthracyclines)• It is classified as an anti-cancer antibiotic (anthracycline type) for adriamycin application.• Anticancer antibiotics (anthracyclines) are drugs that show antitumor effects by inhibiting the synthesis of DNA and RNA necessary for cell proliferation.ADRIACIN Injection 50(アドリアシン注用50)Brand name : ADRIACIN Injection 50 Active ingredient: Doxorubicin hydrochloride Dosage form: injection Print on wrapping:Effects of this medicineThis medicine suppresses cancer cell proliferation by forming a complex with cancer cell DNA and inhibiting DNA and RNA synthetase reactions.It is usually used to improve symptoms of malignant lymphoma, lung cancer, gastrointestinal cancer, breast cancer, bladder tumor or bone sarcoma. It is also used concomitantly with other anti-malignant tumor medicine to treat breast cancer(pre/postoperative chemotherapy in operable patients), uterine body cancer(postoperative chemotherapy, chemotherapy for metastasis/recurrence), malignant tumor of bone/soft tissue , malignant bone tumor, multiple myeloma, childhood malignant solid tumor, urothelial cancer.Before using this medicine, be sure to tell your doctor and pharmacist•If you have previously experienced any allergic reactions (itch, rash, etc.) to any medicines.If you have cardiac dysfunction or a history of it.If you have myelosuppression, liver disorder, renal disorder, infection and chickenpox.•If you are pregnant or breastfeeding.•If you are taking any other medicinal products. (Some medicines may interact to enhance or diminish medicinal effects. Beware of over-the-counter medicines and dietary supplements as well as other prescription medicines.)Dosing schedule (How to take this medicine)•Your dosing schedule prescribed by your doctor is ((to be written by a healthcare professional))•In general, inject this medicine intravenously once a day and then discontinue temporarily. This dosing schedule is repeated. For bladder tumor, inject this medicine intravesically once a day. In any case, duration of administration may vary by other concomitant drug and condition of the patient. Ask your doctor about your dosing schedule.Precautions while taking this medicine• Possible adverse reactions to this medicineThe most commonly reported adverse reactions include alopecia, nausea, vomiting, loss of appetite, stomatitis, anaemia, bladder irritation, fever, contracted bladder, feeling of residual urine, tachycardia, arrhythmia and chest pain. If any of these symptoms occur, consult with your doctor or pharmacist.The symptoms described below are rarely seen as initial symptoms of the adverse reactions indicated in brackets. If any of these symptoms occur, stop taking this medicine and see your doctor immediately.•edema, chest pain, general malaise [myocardial disorder, heart failure]•nasal bleeding, general malaise, fever [myelosuppression such as pancytopenia, anaemia, leukopenia, neutropenia, thrombocytopenia and bleeding]•cold sweat, dizziness, impaired consciousness [shock]•dry cough, shortness of breath, fever [interstitial pneumonia]•urine leakage, frequent urination [contracted bladder]The above symptoms do not describe all the adverse reactions to this medicine. Consult with your doctor or pharmacist if you notice any symptoms of concern other than those listed above.Kyowa Hakko Kirin Co.,LtdInjectionRevised: 12/2014The information on this sheet is based on approvals granted by the Japanese regulatory authority. Approval details may vary by country. Medicines have adverse reactions (risks) as well as efficacies (benefits). It is important to minimize adverse reactions and maximize efficacy. To obtain a better therapeutic response, patients should understand their medication and cooperate with the treatment.用药温馨提示:当您服用此药物时,需定期接受医疗专业人士的检查,以便随时针对其药效、副作用等情况进行监测。本网站所包含的信息旨在为患者提供帮助,不能代替医学建议和治疗。
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 盐酸多柔比星注射剂(ADRIACIN Injection 50mg)
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 盐酸多柔比星注射剂(ADRIACIN Injection 50mg)