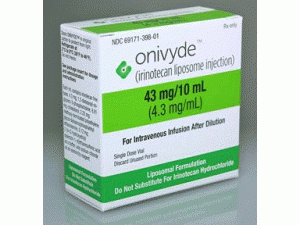
伊立替康脂质体注射剂Onivyde injection 43mg/10mL
 药店国别:
产地国家:美国
处方药:是
所属类别: 43毫克/10毫升(4.3毫克/毫升)/盒
包装规格: 43毫克/10毫升(4.3毫克/毫升)/盒
计价单位:盒
生产厂家中文参考译名:
生产厂家英文名:Merrimack
原产地英文商品名:Onivyde 43mg/10ml(4.3mg/ml)/box
原产地英文药品名:irinotecan liposome injection
中文参考商品译名:Onivyde 43毫克/10毫升(4.3毫克/毫升)/盒
中文参考药品译名:伊立替康脂质体
药店国别:
产地国家:美国
处方药:是
所属类别: 43毫克/10毫升(4.3毫克/毫升)/盒
包装规格: 43毫克/10毫升(4.3毫克/毫升)/盒
计价单位:盒
生产厂家中文参考译名:
生产厂家英文名:Merrimack
原产地英文商品名:Onivyde 43mg/10ml(4.3mg/ml)/box
原产地英文药品名:irinotecan liposome injection
中文参考商品译名:Onivyde 43毫克/10毫升(4.3毫克/毫升)/盒
中文参考药品译名:伊立替康脂质体
简介
近日,美国FDA批准伊立替康脂质体注射液(Onivyde)与氟二氧嘧啶及甲酰四氢叶酸合并用于治疗既往以吉西他滨为基础化疗药物治疗过的晚期(转移性)胰腺癌患者。DA药物评价与研究中心血液学及肿瘤产品办公室主任、医学博士Pazdur称。通过授予伊立替康上市申请优先审评资格,患者将能更早地获取一款有助于延长生存期的药物。批准日期:2015年10月27日 公司:Ipsen Biopharmaceuticals,IncONIVYDE(伊立替康脂质体[irinotecan liposome])注射液,用于静脉注射最初的美国批准:1996年 警告: 严重的新生儿和严重的腹泻.查看完整的盒装警告的完整处方信息•接受ONIVYDE治疗的患者中有0.8%发生致命的中性粒细胞减少性败血症。严重或危及生命的中性粒细胞减少性发热或脓毒症发生率为3%,20%接受ONIVYDEin与氟尿嘧啶和亚叶酸钙联合治疗的患者发生严重威胁生命的中性粒细胞减少症。扣留ONIVYDE的绝对嗜中性粒细胞计数低于1500/mm3或中性粒细胞减少症。 在治疗期间定期监测血细胞计数。•接受ONIVYDE联合氟尿嘧啶和亚叶酸钙治疗的患者中有13%发生严重腹泻。不要给患有肠梗阻的患者服用ONIVYDE。扣留ONIVYDE治疗2-4级严重腹泻。给予洛哌丁胺治疗任何严重程度的晚期腹泻。如果不是禁忌,给予阿托品治疗任何严重程度的早期腹泻。 作用机制 伊立替康脂质体注射剂是包封在脂质双层囊泡或脂质体中的拓扑异构酶1抑制剂。 拓扑异构酶1通过诱导单链断裂减轻DNA中的扭转应变。伊立替康及其活性代谢产物SN-38可逆地结合至异构酶1-DNA复合物并防止单链断裂的重新连接,导致暴露时间依赖性双链DNA损伤和细胞死亡。在携带肿瘤异种移植物的小鼠中,伊立替康脂质体以伊立替康HCl当量剂量施用比伊立替康HCl低5倍,实现了SN-38的类似肿瘤内暴露。 适应症和用法 ONIVYDE是一种拓扑异构酶抑制剂,与氟尿嘧啶和甲酰四氢叶酸组合,用于治疗基于吉西他滨治疗后疾病进展的胰腺转移性腺癌患者。 使用限制 ONIVYDE不作为治疗胰腺转移性腺癌患者的单一药物。 剂量和给药 •不要用ONIVYDE替代含有伊立替康盐酸盐的其他药物。 •推荐剂量的ONIVYDE在每两周90分钟内静脉输注70mg/m2。•对于UGT1A1*28纯合子患者,推荐的ONIVYDE起始剂量为每两周50mg/m2。 •对于血清胆红素高于正常上限的患者,没有推荐剂量的ONIVYDE。 •使用皮质类固醇和止吐药进行预防。在ONIVYDE前30分钟。 剂量形式和强度注射:43mg/10mL单剂量小瓶 禁忌症:对ONIVYDE或盐酸伊立替康的严重超敏反应。 警告和注意事项 •间质性肺病(ILD):接受伊立替康HCl的患者发生致命的ILD。如果ILD被诊断,则停止ONIVYDE。 •严重的超敏反应:永久性地停用ONIVYDE进行严重的超敏反应。 •胚胎-胎儿毒性:可导致胎儿伤害。告知女性有可能对胎儿造成潜在风险并使用有效避孕措施。 不良反应 最常见的不良反应(≥20%)ONIVYDE:腹泻,疲劳/虚弱,呕吐,恶心,食欲减退,口腔炎和发热。最常见的实验室异常(≥3%3级或4级)是淋巴细胞减少和中性粒细胞减少。 药物相互作用 •强CYP3A4诱导剂:尽可能避免使用强CYP3A4诱导剂。在启动ASEIVYDE前至少2周替代非酶诱导疗法。•强CYP3A4抑制剂:尽可能避免使用强CYP3A4或UGT1A1抑制剂;在开始前至少1周停用强CYP3A4抑制剂治疗。 用于特定人群 •哺乳期:不要母乳喂养。包装提供/存储和处理如何提供ONIVYDE有单剂量小瓶,含有43毫克伊立替康游离碱,浓度为4.3毫克/毫升NDC:15054-0043-1 存储和处理 将ONIVYDE储存在2ºC至8ºC(36°F至46°F)。不要冻结。避光。ONIVYDE是一种细胞毒性药物。 遵循适用的特殊处理和处理程序。英文版说明书
INDICATION AND IMPORTANT SAFETY INFORMATIONINDICATIONONIVYDE®(irinotecan liposome injection) is indicated, in combination with fluorouracil (5-FU) and leucovorin (LV), for the treatment of patients with metastatic adenocarcinoma of the pancreas after disease progression following gemcitabine-based therapy.Limitation of Use: ONIVYDE is not indicated as a single agent for the treatment of patients with metastatic adenocarcinoma of the pancreas.WARNING: SEVERE NEUTROPENIA AND SEVERE DIARRHEAFatal neutropenic sepsis occurred in 0.8% of patients receiving ONIVYDE. Severe or life-threatening neutropenic fever or sepsis occurred in 3% and severe or life-threatening neutropenia occurred in 20% of patients receiving ONIVYDE in combination with 5-FU and LV. Withhold ONIVYDE for absolute neutrophil count below 1500/mm3 or neutropenic fever. Monitor blood cell counts periodically during treatmentSevere diarrhea occurred in 13% of patients receiving ONIVYDE in combination with 5-FU/LV. Do not administer ONIVYDE to patients with bowel obstruction. Withhold ONIVYDE for diarrhea of Grade 2–4 severity. Administer loperamide for late diarrhea of any severity. Administer atropine, if not contraindicated, for early diarrhea of any severityCONTRAINDICATIONONIVYDE is contraindicated in patients who have experienced a severe hypersensitivity reaction to ONIVYDE or irinotecan HClWARNINGS AND PRECAUTIONSSevere Neutropenia: See Boxed WARNING. In patients receiving ONIVYDE/ 5-FU/LV, the incidence of Grade 3/4 neutropenia was higher among Asian (18/33 [55%]) vs White patients (13/73 [18%]). Neutropenic fever/neutropenic sepsis was reported in 6% of Asian vs 1% of White patientsSevere Diarrhea: See Boxed WARNING. Severe and life-threatening late-onset (onset >24 hours after chemotherapy [9%]) and early-onset diarrhea (onset ≤24 hours after chemotherapy [3%], sometimes with other symptoms of cholinergic reaction) were observedInterstitial Lung Disease (ILD): Irinotecan HCl can cause severe and fatal ILD. Withhold ONIVYDE in patients with new or progressive dyspnea, cough, and fever, pending diagnostic eva luation. Discontinue ONIVYDE in patients with a confirmed diagnosis of ILDSevere Hypersensitivity Reactions: Irinotecan HCl can cause severe hypersensitivity reactions, including anaphylactic reactions. Permanently discontinue ONIVYDE in patients who experience a severe hypersensitivity reactionEmbryo-Fetal Toxicity: ONIVYDE can cause fetal harm when administered to a pregnant woman. Advise females of reproductive potential to use effective contraception during and for 1 month after ONIVYDE treatmentADVERSE REACTIONSThe most common adverse reactions (≥20%) were diarrhea (59%), fatigue/asthenia (56%), vomiting (52%), nausea (51%), decreased appetite (44%), stomatitis (32%), and pyrexia (23%)The most common Grade 3/4 adverse reactions (≥10%) were diarrhea (13%), fatigue/asthenia (21%), and vomiting (11%)Adverse reactions led to permanent discontinuation of ONIVYDE in 11% of patients receiving ONIVYDE/5-FU/LV; The most frequent adverse reactions resulting in discontinuation of ONIVYDE were diarrhea, vomiting, and sepsisDose reductions of ONIVYDE for adverse reactions occurred in 33% of patients receiving ONIVYDE/5-FU/LV; the most frequent adverse reactions requiring dose reductions were neutropenia, diarrhea, nausea, and anemiaONIVYDE was withheld or delayed for adverse reactions in 62% of patients receiving ONIVYDE/5-FU/LV; the most frequent adverse reactions requiring interruption or delays were neutropenia, diarrhea, fatigue, vomiting, and thrombocytopeniaThe most common laboratory abnormalities (≥20%) were anemia (97%), lymphopenia (81%), neutropenia (52%), increased ALT (51%), hypoalbuminemia (43%), thrombocytopenia (41%), hypomagnesemia (35%), hypokalemia (32%), hypocalcemia (32%), hypophosphatemia (29%), and hyponatremia (27%)DRUG INTERACTIONSAvoid the use of strong CYP3A4 inducers, if possible, and substitute non-enzyme inducing therapies ≥2 weeks prior to initiation of ONIVYDEAvoid the use of strong CYP3A4 or UGT1A1 inhibitors, if possible, and discontinue strong CYP3A4 inhibitors ≥1 week prior to starting therapyUSE IN SPECIFIC POPULATIONSPregnancy and Reproductive Potential: See WARNINGS & PRECAUTIONS. Advise males with female partners of reproductive potential to use condoms during and for 4 months after ONIVYDE treatmentLactation: Advise nursing women not to breastfeed during and for 1 month after ONIVYDE用药温馨提示:当您服用此药物时,需定期接受医疗专业人士的检查,以便随时针对其药效、副作用等情况进行监测。本网站所包含的信息旨在为患者提供帮助,不能代替医学建议和治疗。
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 伊立替康脂质体注射剂Onivyde injection 43mg/10mL
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 伊立替康脂质体注射剂Onivyde injection 43mg/10mL