低分子右旋糖酐_Dextran_低分子右旋糖酐静脉注射说明书
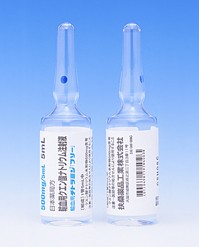
[caption id="attachment_43094" align="alignleft" width="300"] LOW MOLECULAR DEXTRAN D INJECTION(低分子右旋糖酐静脉注射)[/caption]
药店国别:
产地国家:日本
处 方 药:是
所属类别:500毫升/袋 20袋/盒
包装规格:500毫升/袋 20袋/盒
计价单位:盒
生产厂家中文参考译名:大冢制药
生产厂家英文名:Otsuka Pharmaceutical
原产地英文商品名:LOW MOLECULAR DEXTRAN D INJECTION 500ml/bag 20bag/box
原产地英文药品名:Dextran
中文参考商品译名:LMD注射剂 500毫升/袋 20袋/盒
中文参考药品译名:低分子右旋糖酐
简介:
部份中文低分子右旋糖酐 D处方资料(仅供参考)
治疗类别名称:血浆增容,体外循环灌注溶液(低分子量葡聚糖乳酸林格氏液)
商標名:LOW MOLECULAR DEXTRAN D INJECTION
分子量:約40,000(平均分子量)
性状:在白色非晶体粉末,没有气味和味道。乙醇(95)或在乙醚中几乎不溶。逐渐溶解在水中。它是吸湿性的。
操作注意事项:
1.一种用于局部富集由于贮存期间温度变化,这可能是很少沉淀不溶葡聚糖(鳞片状或缩合物)。
2.如果看到不溶性葡聚糖的沉淀,你不要用这个。
3.为了防止此类情况的发生,可以存储在下面的章节“存储”。
4.针点刺直东西○橡胶塞的标志。通过在容器颈部角刺注射针,可以是泄漏的原因。
5.软袋产品不能使用的连接管原理串联系统施用。
6.东西和内容液态水滴被发现是不希望使用被着色或在包装浑浊的之一。
7.容器的液体分至被用作粗略指导。
存储(储存条件):
在室温下储存。但是,为了防止不溶性葡聚糖的沉淀,要注意以下几点。
(1)它被存储在与小温度变化的地方。
例如,冷却器等的出口附近时,应在该温度变化的显著位置避免存储。
(2)不溶性葡聚糖,橡胶塞和容器的软袋内壁和附近7俯卧槽的接触),沉淀尝试在横向载荷的状态保存。
(3)以降低温度变化的影响,其包围软袋外袋优选为未打开。
适应病:
急性出血的治疗作为血浆代用品,特别是有效的急性大量出血的情况下,初始治疗
创伤,烧伤,基于这样的手术出血休克的预防和治疗
在手术时输血量的储蓄
它用作体外循环灌注溶液,以减少手术期间的并发症的危险性,以促进灌注。
用法用量:
静脉注射一次500毫升慢。
此外,年龄,体重,适当增加或根据状况减少。
当用作体外循环灌注溶液用于注入2〜3G(20〜30毫升),为每右旋糖酐40公斤体重。
包装规格:低分子右旋糖酐 500毫升Tochu 20袋含有软袋
制造厂商:大冢制药有限公司
Therapeutic category name
Blood flow improvement and extracorporeal circulation perfusion solution (glucose addition dextran 40 injection)
Brand Name
Low molecular weight dextran Tochu
composition
The agent is injected solution containing the following ingredients in a first container (500 mL).
Dextran 40
50g
Glucose
25g
Contraindication
Hypotonic dehydration of the patient [this disease is caused by the osmotic pressure of the serum is hypotonic by the lack of sodium. Administration of this drug such a patient, results in increasing the water content, there is a risk that symptoms worse. ]
A burden to the heart from the fact that increasing the patients' circulating blood volume with congestive heart failure, there is a possibility that the symptoms worse. ]
Efficacy or effect
Treatment of bleeding and this by the resulting shock
Savings of blood transfusion during surgery
Prevention and treatment of thrombosis
Peripheral blood circulation improvement at the time of trauma, burns, fractures, and the like, and severe shock
It used as an extracorporeal circulation perfusion solution, to reduce the risk of complications during surgery to facilitate the perfusion.
A normal adult once 500mL intravenous injection.
The dosage of the first 24 hours is equal to or less than 20mL / kg.
When continuous administration as the prevention and treatment of thrombosis, and less than 1 day 10mL / kg, and within five days.
The extracorporeal circulation perfusion fluid, is injected 10~20mL / kg.
However, injection amount is equal to or less than 20mL / kg.
In addition, dose, dose rate is age, body weight, appropriately increased or decreased depending on the condition.
To avoid long-term use (kept in as much as possible short-term administration, and within 5 days).
Careful administration
There is a risk of worsening the patient [renal failure with renal failure. ]
There is a risk that the incentive of a patient's state of dehydration [renal dysfunction expression. ]
Of pulmonary edema patients [moisture is retained in the lung cell stroma, there is a possibility that the pulmonary edema is worsening. ]
Hypofibrinogenemia, there is a fear that by suppressing the patient [coagulation system with a bleeding tendency, such as thrombocytopenia to promote the bleeding tendency. ]
Since the transition to diabetes patients [glucose tissue is suppressed, which may symptoms occur hyperglycemia is deteriorated. ]
Potassium by the administration of patients' glucose with potassium deficiency tendency to migrate into the cell, temporarily serum potassium level is lowered, there is a possibility that the symptoms worse. ]
Diabetes insipidus patient [proper moisture in this disease, it is necessary electrolyte management, affecting the electrolyte or the like by the administration of this drug, there is a risk that symptoms worse. ]
Clinically significant adverse reactions
shock
(Frequency unknown)
Because it may cause shock symptoms, carefully monitored, lowering blood pressure, pulse rate of abnormalities, discontinue immediately administration if the symptoms such as respiratory depression appeared, to carry out the symptomatic treatment (first-order re-eva luation a result that 16, 1979).
Acute renal failure
(Frequency unknown)
Since acute renal failure may occur, if the oliguria such abnormalities are observed, discontinue administration, continuous blood filtration dialysis, plasma exchange, to carry out the appropriate treatment of blood dialysis, etc. (primary re-eva luation result that 16, 1979).
Hypersensitivity
(Frequency unknown)
If hypersensitivity symptoms such as anaphylaxis appeared administration should be discontinued and a symptomatic treatment (voluntary revised, 1993).
Pharmacology
Peripheral circulation blood flow improving action
Hemagglutination dissociation action
In experiments in vitro using human red blood cells, 1 dextran 40 to enhance the negative charge of the red blood cell surface, it showed a red blood cell aggregation dissociation action).
Further, Dextran 40 dissociates the intravascular hemagglutination formed in the liver of the rabbits were improved peripheral blood flow 2).
Lowering effect blood viscosity
Dextran 40 reduced the blood viscosity postoperative shock patient 3).
Blood pressure effect of maintaining
DatsuchiNaru a result of the administration of this drug to the dog, blood pressure, 4 heart rate, improved the effect of maintaining the crotch arterial blood flow was observed).
Thromboprophylaxis effect
In experiments with sheep that created both the femoral artery thrombosis by energization method, dextran 40 showed excellent blood clot prevention and dissolution effect 5).
Physicochemical knowledge of active ingredient
Generic name
Dextran 40
Molecular weight
About 40,000 (average molecular weight)
Behavior
In non-crystalline powder of white, there is no smell and taste. Ethanol (95) or practically insoluble in diethyl ether. Gradually dissolved in water. It is hygroscopic.
LOW MOLECULAR DEXTRAN D INJECTION(低分子右旋糖酐静脉注射)[/caption]
药店国别:
产地国家:日本
处 方 药:是
所属类别:500毫升/袋 20袋/盒
包装规格:500毫升/袋 20袋/盒
计价单位:盒
生产厂家中文参考译名:大冢制药
生产厂家英文名:Otsuka Pharmaceutical
原产地英文商品名:LOW MOLECULAR DEXTRAN D INJECTION 500ml/bag 20bag/box
原产地英文药品名:Dextran
中文参考商品译名:LMD注射剂 500毫升/袋 20袋/盒
中文参考药品译名:低分子右旋糖酐
简介:
部份中文低分子右旋糖酐 D处方资料(仅供参考)
治疗类别名称:血浆增容,体外循环灌注溶液(低分子量葡聚糖乳酸林格氏液)
商標名:LOW MOLECULAR DEXTRAN D INJECTION
分子量:約40,000(平均分子量)
性状:在白色非晶体粉末,没有气味和味道。乙醇(95)或在乙醚中几乎不溶。逐渐溶解在水中。它是吸湿性的。
操作注意事项:
1.一种用于局部富集由于贮存期间温度变化,这可能是很少沉淀不溶葡聚糖(鳞片状或缩合物)。
2.如果看到不溶性葡聚糖的沉淀,你不要用这个。
3.为了防止此类情况的发生,可以存储在下面的章节“存储”。
4.针点刺直东西○橡胶塞的标志。通过在容器颈部角刺注射针,可以是泄漏的原因。
5.软袋产品不能使用的连接管原理串联系统施用。
6.东西和内容液态水滴被发现是不希望使用被着色或在包装浑浊的之一。
7.容器的液体分至被用作粗略指导。
存储(储存条件):
在室温下储存。但是,为了防止不溶性葡聚糖的沉淀,要注意以下几点。
(1)它被存储在与小温度变化的地方。
例如,冷却器等的出口附近时,应在该温度变化的显著位置避免存储。
(2)不溶性葡聚糖,橡胶塞和容器的软袋内壁和附近7俯卧槽的接触),沉淀尝试在横向载荷的状态保存。
(3)以降低温度变化的影响,其包围软袋外袋优选为未打开。
适应病:
急性出血的治疗作为血浆代用品,特别是有效的急性大量出血的情况下,初始治疗
创伤,烧伤,基于这样的手术出血休克的预防和治疗
在手术时输血量的储蓄
它用作体外循环灌注溶液,以减少手术期间的并发症的危险性,以促进灌注。
用法用量:
静脉注射一次500毫升慢。
此外,年龄,体重,适当增加或根据状况减少。
当用作体外循环灌注溶液用于注入2〜3G(20〜30毫升),为每右旋糖酐40公斤体重。
包装规格:低分子右旋糖酐 500毫升Tochu 20袋含有软袋
制造厂商:大冢制药有限公司
Therapeutic category name
Blood flow improvement and extracorporeal circulation perfusion solution (glucose addition dextran 40 injection)
Brand Name
Low molecular weight dextran Tochu
composition
The agent is injected solution containing the following ingredients in a first container (500 mL).
Dextran 40
50g
Glucose
25g
Contraindication
Hypotonic dehydration of the patient [this disease is caused by the osmotic pressure of the serum is hypotonic by the lack of sodium. Administration of this drug such a patient, results in increasing the water content, there is a risk that symptoms worse. ]
A burden to the heart from the fact that increasing the patients' circulating blood volume with congestive heart failure, there is a possibility that the symptoms worse. ]
Efficacy or effect
Treatment of bleeding and this by the resulting shock
Savings of blood transfusion during surgery
Prevention and treatment of thrombosis
Peripheral blood circulation improvement at the time of trauma, burns, fractures, and the like, and severe shock
It used as an extracorporeal circulation perfusion solution, to reduce the risk of complications during surgery to facilitate the perfusion.
A normal adult once 500mL intravenous injection.
The dosage of the first 24 hours is equal to or less than 20mL / kg.
When continuous administration as the prevention and treatment of thrombosis, and less than 1 day 10mL / kg, and within five days.
The extracorporeal circulation perfusion fluid, is injected 10~20mL / kg.
However, injection amount is equal to or less than 20mL / kg.
In addition, dose, dose rate is age, body weight, appropriately increased or decreased depending on the condition.
To avoid long-term use (kept in as much as possible short-term administration, and within 5 days).
Careful administration
There is a risk of worsening the patient [renal failure with renal failure. ]
There is a risk that the incentive of a patient's state of dehydration [renal dysfunction expression. ]
Of pulmonary edema patients [moisture is retained in the lung cell stroma, there is a possibility that the pulmonary edema is worsening. ]
Hypofibrinogenemia, there is a fear that by suppressing the patient [coagulation system with a bleeding tendency, such as thrombocytopenia to promote the bleeding tendency. ]
Since the transition to diabetes patients [glucose tissue is suppressed, which may symptoms occur hyperglycemia is deteriorated. ]
Potassium by the administration of patients' glucose with potassium deficiency tendency to migrate into the cell, temporarily serum potassium level is lowered, there is a possibility that the symptoms worse. ]
Diabetes insipidus patient [proper moisture in this disease, it is necessary electrolyte management, affecting the electrolyte or the like by the administration of this drug, there is a risk that symptoms worse. ]
Clinically significant adverse reactions
shock
(Frequency unknown)
Because it may cause shock symptoms, carefully monitored, lowering blood pressure, pulse rate of abnormalities, discontinue immediately administration if the symptoms such as respiratory depression appeared, to carry out the symptomatic treatment (first-order re-eva luation a result that 16, 1979).
Acute renal failure
(Frequency unknown)
Since acute renal failure may occur, if the oliguria such abnormalities are observed, discontinue administration, continuous blood filtration dialysis, plasma exchange, to carry out the appropriate treatment of blood dialysis, etc. (primary re-eva luation result that 16, 1979).
Hypersensitivity
(Frequency unknown)
If hypersensitivity symptoms such as anaphylaxis appeared administration should be discontinued and a symptomatic treatment (voluntary revised, 1993).
Pharmacology
Peripheral circulation blood flow improving action
Hemagglutination dissociation action
In experiments in vitro using human red blood cells, 1 dextran 40 to enhance the negative charge of the red blood cell surface, it showed a red blood cell aggregation dissociation action).
Further, Dextran 40 dissociates the intravascular hemagglutination formed in the liver of the rabbits were improved peripheral blood flow 2).
Lowering effect blood viscosity
Dextran 40 reduced the blood viscosity postoperative shock patient 3).
Blood pressure effect of maintaining
DatsuchiNaru a result of the administration of this drug to the dog, blood pressure, 4 heart rate, improved the effect of maintaining the crotch arterial blood flow was observed).
Thromboprophylaxis effect
In experiments with sheep that created both the femoral artery thrombosis by energization method, dextran 40 showed excellent blood clot prevention and dissolution effect 5).
Physicochemical knowledge of active ingredient
Generic name
Dextran 40
Molecular weight
About 40,000 (average molecular weight)
Behavior
In non-crystalline powder of white, there is no smell and taste. Ethanol (95) or practically insoluble in diethyl ether. Gradually dissolved in water. It is hygroscopic.
 LOW MOLECULAR DEXTRAN D INJECTION(低分子右旋糖酐静脉注射)[/caption]
药店国别:
产地国家:日本
处 方 药:是
所属类别:500毫升/袋 20袋/盒
包装规格:500毫升/袋 20袋/盒
计价单位:盒
生产厂家中文参考译名:大冢制药
生产厂家英文名:Otsuka Pharmaceutical
原产地英文商品名:LOW MOLECULAR DEXTRAN D INJECTION 500ml/bag 20bag/box
原产地英文药品名:Dextran
中文参考商品译名:LMD注射剂 500毫升/袋 20袋/盒
中文参考药品译名:低分子右旋糖酐
简介:
部份中文低分子右旋糖酐 D处方资料(仅供参考)
治疗类别名称:血浆增容,体外循环灌注溶液(低分子量葡聚糖乳酸林格氏液)
商標名:LOW MOLECULAR DEXTRAN D INJECTION
分子量:約40,000(平均分子量)
性状:在白色非晶体粉末,没有气味和味道。乙醇(95)或在乙醚中几乎不溶。逐渐溶解在水中。它是吸湿性的。
操作注意事项:
1.一种用于局部富集由于贮存期间温度变化,这可能是很少沉淀不溶葡聚糖(鳞片状或缩合物)。
2.如果看到不溶性葡聚糖的沉淀,你不要用这个。
3.为了防止此类情况的发生,可以存储在下面的章节“存储”。
4.针点刺直东西○橡胶塞的标志。通过在容器颈部角刺注射针,可以是泄漏的原因。
5.软袋产品不能使用的连接管原理串联系统施用。
6.东西和内容液态水滴被发现是不希望使用被着色或在包装浑浊的之一。
7.容器的液体分至被用作粗略指导。
存储(储存条件):
在室温下储存。但是,为了防止不溶性葡聚糖的沉淀,要注意以下几点。
(1)它被存储在与小温度变化的地方。
例如,冷却器等的出口附近时,应在该温度变化的显著位置避免存储。
(2)不溶性葡聚糖,橡胶塞和容器的软袋内壁和附近7俯卧槽的接触),沉淀尝试在横向载荷的状态保存。
(3)以降低温度变化的影响,其包围软袋外袋优选为未打开。
适应病:
急性出血的治疗作为血浆代用品,特别是有效的急性大量出血的情况下,初始治疗
创伤,烧伤,基于这样的手术出血休克的预防和治疗
在手术时输血量的储蓄
它用作体外循环灌注溶液,以减少手术期间的并发症的危险性,以促进灌注。
用法用量:
静脉注射一次500毫升慢。
此外,年龄,体重,适当增加或根据状况减少。
当用作体外循环灌注溶液用于注入2〜3G(20〜30毫升),为每右旋糖酐40公斤体重。
包装规格:低分子右旋糖酐 500毫升Tochu 20袋含有软袋
制造厂商:大冢制药有限公司
Therapeutic category name
Blood flow improvement and extracorporeal circulation perfusion solution (glucose addition dextran 40 injection)
Brand Name
Low molecular weight dextran Tochu
composition
The agent is injected solution containing the following ingredients in a first container (500 mL).
Dextran 40
50g
Glucose
25g
Contraindication
Hypotonic dehydration of the patient [this disease is caused by the osmotic pressure of the serum is hypotonic by the lack of sodium. Administration of this drug such a patient, results in increasing the water content, there is a risk that symptoms worse. ]
A burden to the heart from the fact that increasing the patients' circulating blood volume with congestive heart failure, there is a possibility that the symptoms worse. ]
Efficacy or effect
Treatment of bleeding and this by the resulting shock
Savings of blood transfusion during surgery
Prevention and treatment of thrombosis
Peripheral blood circulation improvement at the time of trauma, burns, fractures, and the like, and severe shock
It used as an extracorporeal circulation perfusion solution, to reduce the risk of complications during surgery to facilitate the perfusion.
A normal adult once 500mL intravenous injection.
The dosage of the first 24 hours is equal to or less than 20mL / kg.
When continuous administration as the prevention and treatment of thrombosis, and less than 1 day 10mL / kg, and within five days.
The extracorporeal circulation perfusion fluid, is injected 10~20mL / kg.
However, injection amount is equal to or less than 20mL / kg.
In addition, dose, dose rate is age, body weight, appropriately increased or decreased depending on the condition.
To avoid long-term use (kept in as much as possible short-term administration, and within 5 days).
Careful administration
There is a risk of worsening the patient [renal failure with renal failure. ]
There is a risk that the incentive of a patient's state of dehydration [renal dysfunction expression. ]
Of pulmonary edema patients [moisture is retained in the lung cell stroma, there is a possibility that the pulmonary edema is worsening. ]
Hypofibrinogenemia, there is a fear that by suppressing the patient [coagulation system with a bleeding tendency, such as thrombocytopenia to promote the bleeding tendency. ]
Since the transition to diabetes patients [glucose tissue is suppressed, which may symptoms occur hyperglycemia is deteriorated. ]
Potassium by the administration of patients' glucose with potassium deficiency tendency to migrate into the cell, temporarily serum potassium level is lowered, there is a possibility that the symptoms worse. ]
Diabetes insipidus patient [proper moisture in this disease, it is necessary electrolyte management, affecting the electrolyte or the like by the administration of this drug, there is a risk that symptoms worse. ]
Clinically significant adverse reactions
shock
(Frequency unknown)
Because it may cause shock symptoms, carefully monitored, lowering blood pressure, pulse rate of abnormalities, discontinue immediately administration if the symptoms such as respiratory depression appeared, to carry out the symptomatic treatment (first-order re-eva luation a result that 16, 1979).
Acute renal failure
(Frequency unknown)
Since acute renal failure may occur, if the oliguria such abnormalities are observed, discontinue administration, continuous blood filtration dialysis, plasma exchange, to carry out the appropriate treatment of blood dialysis, etc. (primary re-eva luation result that 16, 1979).
Hypersensitivity
(Frequency unknown)
If hypersensitivity symptoms such as anaphylaxis appeared administration should be discontinued and a symptomatic treatment (voluntary revised, 1993).
Pharmacology
Peripheral circulation blood flow improving action
Hemagglutination dissociation action
In experiments in vitro using human red blood cells, 1 dextran 40 to enhance the negative charge of the red blood cell surface, it showed a red blood cell aggregation dissociation action).
Further, Dextran 40 dissociates the intravascular hemagglutination formed in the liver of the rabbits were improved peripheral blood flow 2).
Lowering effect blood viscosity
Dextran 40 reduced the blood viscosity postoperative shock patient 3).
Blood pressure effect of maintaining
DatsuchiNaru a result of the administration of this drug to the dog, blood pressure, 4 heart rate, improved the effect of maintaining the crotch arterial blood flow was observed).
Thromboprophylaxis effect
In experiments with sheep that created both the femoral artery thrombosis by energization method, dextran 40 showed excellent blood clot prevention and dissolution effect 5).
Physicochemical knowledge of active ingredient
Generic name
Dextran 40
Molecular weight
About 40,000 (average molecular weight)
Behavior
In non-crystalline powder of white, there is no smell and taste. Ethanol (95) or practically insoluble in diethyl ether. Gradually dissolved in water. It is hygroscopic.
LOW MOLECULAR DEXTRAN D INJECTION(低分子右旋糖酐静脉注射)[/caption]
药店国别:
产地国家:日本
处 方 药:是
所属类别:500毫升/袋 20袋/盒
包装规格:500毫升/袋 20袋/盒
计价单位:盒
生产厂家中文参考译名:大冢制药
生产厂家英文名:Otsuka Pharmaceutical
原产地英文商品名:LOW MOLECULAR DEXTRAN D INJECTION 500ml/bag 20bag/box
原产地英文药品名:Dextran
中文参考商品译名:LMD注射剂 500毫升/袋 20袋/盒
中文参考药品译名:低分子右旋糖酐
简介:
部份中文低分子右旋糖酐 D处方资料(仅供参考)
治疗类别名称:血浆增容,体外循环灌注溶液(低分子量葡聚糖乳酸林格氏液)
商標名:LOW MOLECULAR DEXTRAN D INJECTION
分子量:約40,000(平均分子量)
性状:在白色非晶体粉末,没有气味和味道。乙醇(95)或在乙醚中几乎不溶。逐渐溶解在水中。它是吸湿性的。
操作注意事项:
1.一种用于局部富集由于贮存期间温度变化,这可能是很少沉淀不溶葡聚糖(鳞片状或缩合物)。
2.如果看到不溶性葡聚糖的沉淀,你不要用这个。
3.为了防止此类情况的发生,可以存储在下面的章节“存储”。
4.针点刺直东西○橡胶塞的标志。通过在容器颈部角刺注射针,可以是泄漏的原因。
5.软袋产品不能使用的连接管原理串联系统施用。
6.东西和内容液态水滴被发现是不希望使用被着色或在包装浑浊的之一。
7.容器的液体分至被用作粗略指导。
存储(储存条件):
在室温下储存。但是,为了防止不溶性葡聚糖的沉淀,要注意以下几点。
(1)它被存储在与小温度变化的地方。
例如,冷却器等的出口附近时,应在该温度变化的显著位置避免存储。
(2)不溶性葡聚糖,橡胶塞和容器的软袋内壁和附近7俯卧槽的接触),沉淀尝试在横向载荷的状态保存。
(3)以降低温度变化的影响,其包围软袋外袋优选为未打开。
适应病:
急性出血的治疗作为血浆代用品,特别是有效的急性大量出血的情况下,初始治疗
创伤,烧伤,基于这样的手术出血休克的预防和治疗
在手术时输血量的储蓄
它用作体外循环灌注溶液,以减少手术期间的并发症的危险性,以促进灌注。
用法用量:
静脉注射一次500毫升慢。
此外,年龄,体重,适当增加或根据状况减少。
当用作体外循环灌注溶液用于注入2〜3G(20〜30毫升),为每右旋糖酐40公斤体重。
包装规格:低分子右旋糖酐 500毫升Tochu 20袋含有软袋
制造厂商:大冢制药有限公司
Therapeutic category name
Blood flow improvement and extracorporeal circulation perfusion solution (glucose addition dextran 40 injection)
Brand Name
Low molecular weight dextran Tochu
composition
The agent is injected solution containing the following ingredients in a first container (500 mL).
Dextran 40
50g
Glucose
25g
Contraindication
Hypotonic dehydration of the patient [this disease is caused by the osmotic pressure of the serum is hypotonic by the lack of sodium. Administration of this drug such a patient, results in increasing the water content, there is a risk that symptoms worse. ]
A burden to the heart from the fact that increasing the patients' circulating blood volume with congestive heart failure, there is a possibility that the symptoms worse. ]
Efficacy or effect
Treatment of bleeding and this by the resulting shock
Savings of blood transfusion during surgery
Prevention and treatment of thrombosis
Peripheral blood circulation improvement at the time of trauma, burns, fractures, and the like, and severe shock
It used as an extracorporeal circulation perfusion solution, to reduce the risk of complications during surgery to facilitate the perfusion.
A normal adult once 500mL intravenous injection.
The dosage of the first 24 hours is equal to or less than 20mL / kg.
When continuous administration as the prevention and treatment of thrombosis, and less than 1 day 10mL / kg, and within five days.
The extracorporeal circulation perfusion fluid, is injected 10~20mL / kg.
However, injection amount is equal to or less than 20mL / kg.
In addition, dose, dose rate is age, body weight, appropriately increased or decreased depending on the condition.
To avoid long-term use (kept in as much as possible short-term administration, and within 5 days).
Careful administration
There is a risk of worsening the patient [renal failure with renal failure. ]
There is a risk that the incentive of a patient's state of dehydration [renal dysfunction expression. ]
Of pulmonary edema patients [moisture is retained in the lung cell stroma, there is a possibility that the pulmonary edema is worsening. ]
Hypofibrinogenemia, there is a fear that by suppressing the patient [coagulation system with a bleeding tendency, such as thrombocytopenia to promote the bleeding tendency. ]
Since the transition to diabetes patients [glucose tissue is suppressed, which may symptoms occur hyperglycemia is deteriorated. ]
Potassium by the administration of patients' glucose with potassium deficiency tendency to migrate into the cell, temporarily serum potassium level is lowered, there is a possibility that the symptoms worse. ]
Diabetes insipidus patient [proper moisture in this disease, it is necessary electrolyte management, affecting the electrolyte or the like by the administration of this drug, there is a risk that symptoms worse. ]
Clinically significant adverse reactions
shock
(Frequency unknown)
Because it may cause shock symptoms, carefully monitored, lowering blood pressure, pulse rate of abnormalities, discontinue immediately administration if the symptoms such as respiratory depression appeared, to carry out the symptomatic treatment (first-order re-eva luation a result that 16, 1979).
Acute renal failure
(Frequency unknown)
Since acute renal failure may occur, if the oliguria such abnormalities are observed, discontinue administration, continuous blood filtration dialysis, plasma exchange, to carry out the appropriate treatment of blood dialysis, etc. (primary re-eva luation result that 16, 1979).
Hypersensitivity
(Frequency unknown)
If hypersensitivity symptoms such as anaphylaxis appeared administration should be discontinued and a symptomatic treatment (voluntary revised, 1993).
Pharmacology
Peripheral circulation blood flow improving action
Hemagglutination dissociation action
In experiments in vitro using human red blood cells, 1 dextran 40 to enhance the negative charge of the red blood cell surface, it showed a red blood cell aggregation dissociation action).
Further, Dextran 40 dissociates the intravascular hemagglutination formed in the liver of the rabbits were improved peripheral blood flow 2).
Lowering effect blood viscosity
Dextran 40 reduced the blood viscosity postoperative shock patient 3).
Blood pressure effect of maintaining
DatsuchiNaru a result of the administration of this drug to the dog, blood pressure, 4 heart rate, improved the effect of maintaining the crotch arterial blood flow was observed).
Thromboprophylaxis effect
In experiments with sheep that created both the femoral artery thrombosis by energization method, dextran 40 showed excellent blood clot prevention and dissolution effect 5).
Physicochemical knowledge of active ingredient
Generic name
Dextran 40
Molecular weight
About 40,000 (average molecular weight)
Behavior
In non-crystalline powder of white, there is no smell and taste. Ethanol (95) or practically insoluble in diethyl ether. Gradually dissolved in water. It is hygroscopic.
用药温馨提示:当您服用此药物时,需定期接受医疗专业人士的检查,以便随时针对其药效、副作用等情况进行监测。本网站所包含的信息旨在为患者提供帮助,不能代替医学建议和治疗。
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 低分子右旋糖酐_Dextran_低分子右旋糖酐静脉注射说明书
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 低分子右旋糖酐_Dextran_低分子右旋糖酐静脉注射说明书