依前列醇钠_FLOLAN_依前列醇钠注射剂说明书
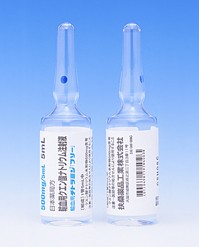
[caption id="attachment_42914" align="alignleft" width="300"] FLOLAN for Injection 0.5mg(依前列醇钠注射剂)[/caption]
药店国别:
产地国家:美国
处 方 药:是
所属类别:0.5毫克/毫升/瓶
包装规格:0.5毫克/毫升/瓶
计价单位:瓶
生产厂家中文参考译名:GlaxoSmithKline LLC
生产厂家英文名:GlaxoSmithKline LLC
原产地英文商品名:FLOLAN 0.5 MG POWDER AND SOLVENT FOR SOLUTION FOR INFUSION
原产地英文药品名:EPOPROSTENOL SODIUM
中文参考商品译名:服疗能 粉剂和溶剂解输液 0.5毫克/毫升/瓶
中文参考药品译名:依前列醇钠
简介:
部份中文依前列醇钠处方资料(仅供参考)
药品英文名:Epoprostenol
药品别名:前列环素、依前列醇钠、环前列烯醇、环依前列烯醇、前列环素I2、PGX、Cycloprostin、PGI-2、Prostacyclin
药物剂型:注射剂(粉)(钠盐):500μg。临用时以pH 10.5的含甘氨酸的专用缓冲剂溶解。
药理作用:本品可通过增加血小板中的cAMP抑制血小板聚集,为已发现抗凝药中最强者。本品通过肺循环时不被代谢,可防止体外循环时血栓形成;较高剂量可使血小板凝块解聚,使皮肤出血时间延长2倍;可降低血小板的促凝作用,并减少其肝素中和因子的释放。本品可抑制ADP、胶原、花生四烯酸等诱导的血小板聚集和释放,且具有解聚作用。
药动学:参见:前列腺素E1
适应证:用于心肺分流术及炭血液灌注时保护血小板功能;也用于肾透析时代替肝素。用于冠状粥样硬化性心脏病不稳定型心绞痛、心肌梗死等。
禁忌证:有出血倾向者禁用。儿童、孕妇、哺乳妇女不宜使用。
注意事项:
1.在心肺分流术或血流灌注时不可代替肝素,仅在肾透析时代替肝素。若透析回路发生凝块,应停止透析。
2.超剂量使用可发生降压,应减量或停药。
不良反应:
1.高剂量时可见血压下降、心搏徐缓、面部潮红、头痛,以及胃痉挛痛、恶心、呕吐、腹部不适等。
2.对自发性或药物性出血者,应考虑引起出血并发症的可能。
用法用量:
1.静脉滴注:成人心肺分流术先每分钟连续静脉滴注10ng/kg;在分流术中每分钟静脉滴注20ng/kg,术毕即停注。
2.炭血灌注:先每分钟静脉滴注2~16ng/kg,灌注时每分钟静脉注射16ng/kg于碳柱的近侧管内。肾透析:透析前每分钟静脉滴注5ng/kg,透析时滴注于透析器的动脉入口。对老年人用剂量尚未验证是否需要修正。
药物相应作用:本品与抗凝剂、血管扩张药以及影响心血管反射的药物并用时有协同作用,需慎重。
专家点评:
本品可通过增加血小板中的cAMP抑制血小板聚集,为已发现的抗凝药中最强者。本品通过肺循环时不被代谢,可防止体外循环时血栓形成,较高剂量可使血小板凝块解聚,延长皮肤出血时间2倍,可降低血小板的促凝作用,并减少其肝素中和因子的释放。
FlolanGeneric Name: epoprostenol (Intravenous route)
e-poe-PROST-en-ol
Commonly used brand name(s):
In the U.S.
Flolan
Available Dosage Forms:
Powder for Solution
Therapeutic Class: Peripheral Vasodilator
Pharmacologic Class: Prostaglandin
Uses For Flolan
Epoprostenol belongs to a group of agents called prostaglandins. Prostaglandins occur naturally in the body and are involved in many biological functions. Epoprostenol is used to treat the symptoms of primary pulmonary hypertension, or the high blood pressure that occurs in the main artery that carries blood from the right side of the heart (the ventricle) to the lungs. When the smaller blood vessels in the lungs become more resistant to blood flow, the right ventricle must work harder to pump enough blood through the lungs. Epoprostenol works by relaxing blood vessels and increasing the supply of blood to the lungs, reducing the workload of the heart.
This medicine is available only with your doctor's prescription.
Before Using Flolan
In deciding to use a medicine, the risks of taking the medicine must be weighed against the good it will do. This is a decision you and your doctor will make. For this medicine, the following should be considered:
Allergies
Tell your doctor if you have ever had any unusual or allergic reaction to this medicine or any other medicines. Also tell your health care professional if you have any other types of allergies, such as to foods, dyes, preservatives, or animals. For non-prescription products, read the label or package ingredients carefully.
Pediatric
Although there is no specific information comparing use of this medicine in children with use in other age groups, this medicine is not expected to cause different side effects or problems in children than it does in adults.
Geriatric
Many medicines have not been studied specifically in older people. Therefore, it may not be known whether they work exactly the same way they do in younger adults or if they cause different side effects or problems in older people. There is no specific information comparing use of epoprostenol in the elderly with use in other age groups.
Pregnancy
Pregnancy Category Explanation
All Trimesters B Animal studies have revealed no evidence of harm to the fetus, however, there are no adequate studies in pregnant women OR animal studies have shown an adverse effect, but adequate studies in pregnant women have failed to demonstrate a risk to the fetus.
Breast Feeding
There are no adequate studies in women for determining infant risk when using this medication during breastfeeding. Weigh the potential benefits against the potential risks before taking this medication while breastfeeding.
Interactions with Medicines
Using this medicine with any of the following medicines is usually not recommended, but may be required in some cases. If both medicines are prescribed together, your doctor may change the dose or how often you use one or both of the medicines.
Desvenlafaxine
Duloxetine
Milnacipran
Venlafaxine
Using this medicine with any of the following medicines may cause an increased risk of certain side effects, but using both drugs may be the best treatment for you. If both medicines are prescribed together, your doctor may change the dose or how often you use one or both of the medicines.
Digoxin
Interactions with Food/Tobacco/Alcohol
Certain medicines should not be used at or around the time of eating food or eating certain types of food since interactions may occur. Using alcohol or tobacco with certain medicines may also cause interactions to occur. Discuss with your healthcare professional the use of your medicine with food, alcohol, or tobacco.
Other Medical Problems
The presence of other medical problems may affect the use of this medicine. Make sure you tell your doctor if you have any other medical problems, especially:
Heart disease or
Lung disease—Epoprostenol may make these conditions worse
Proper Use of Flolan
Your doctor or nurse will teach you how to prepare the medicine and use the pump for administering the medicine. Epoprostenol must be administered continuously by a portable pump that is operated by a small computer. The medicine will be delivered directly to the heart through a catheter that will be inserted into a vein in the chest.
Epoprostenol should be reconstituted only with the sterile diluent that is supplied with this medicine. The reconstituted medicine should not be mixed with other solutions or medicines. Use the following procedure for reconstituting your daily supply:
Clear an area to work in and clean the area with alcohol. Gather your supplies. Wash your hands thoroughly with soap and water and then open all packages. Remove the vial cap from the vial containing the sterile diluent, clean the tops of the vials with alcohol swabs, and let the vial tops dry before proceeding.
To withdraw the sterile diluent
If not already attached, attach a needle to the syringe. Gently pull the plunger out slightly and push it back to break the syringe seal. Draw air into the syringe that is about equal to the amount of sterile diluent you've been instructed to withdraw from the vial. Insert the needle at an angle, completely through the rubber seal of the vial. Turn the vial and syringe upside down (the syringe-vial unit is now vertical) and carefully press the plunger, injecting some or all of the air into the vial. Then aim the tip of the needle into the fluid and carefully pull the plunger slowly back to withdraw the diluent and/or allow the pressure to fill the syringe with the diluent. Continue pushing the remaining air into the vial, allowing the liquid to enter the syringe until the prescribed amount of diluent has been drawn into the syringe. Without withdrawing the needle, tap the syringe gently so that any air bubbles trapped in the syringe rise toward the top of the syringe. If air bubbles appear, depress the plunger gently to force the air bubbles out (into the vial) and then withdraw enough additional diluent to restore the needed volume in the syringe. (Holding the syringe-vial as a unit in a vertical position and keeping the needle tip in the fluid while withdrawing the diluent may help minimize the amount of air drawn into the syringe.) Once the required volume has been drawn into the syringe, let the syringe-vial pressure equalize and slowly withdraw the needle from the vial.
To reconstitute the epoprostenol
Insert the same needle through the rubber seal of the vial of epoprostenol and inject the sterile diluent gently onto the side of the vial. The flow of the sterile diluent should be directed toward the side of the vial and injected slowly in order to prevent the medicine from foaming. Once the pressure has equalized, withdraw the needle from the vial. Gently swirl the vial to mix the epoprostenol. Turn the vial upside down to catch any undissolved powder near the top of the vial. Never shake the vials. Repeat this process if you need to mix more than one vial of epoprostenol.
To draw out the reconstituted epoprostenol
Wipe the top of the reconstituted epoprostenol vial with an alcohol swab and let it dry. Change the needle on the syringe and then gently pull back the syringe plunger and fill the syringe with the amount of air that is equal to the amount of reconstituted epoprostenol you have been instructed to withdraw. Insert the needle through the seal of the vial and inject the air into the vial. Be sure to keep the needle tip below the fluid line and then pull the plunger back gently to withdraw the reconstituted epoprostenol into the syringe. Remove any air that may be trapped in the syringe as described above. Withdraw the needle and replace the needle cap on the syringe.
To inject the reconstituted epoprostenol into the cassette
Remove the end cap from the cassette tubing. Carefully remove the needle from the syringe (be sure to discard the needle in an appropriate manner) and attach the syringe to the cassette tubing. Hold the cassette in one hand and push the plunger to inject the reconstituted solution into the cassette (alternatively, you may find it useful to use a tabletop or other solid structure to steady the plunger while pushing down on the syringe to inject the solution). When the syringe is empty, clamp the cassette tubing near the syringe. Disconnect the syringe and replace the cassette tubing end cap.
To inject the remaining diluent into the partially filled cassette
Using a 60 mL syringe, attach a new needle to the syringe and follow the above procedures for breaking the syringe seal and wiping the tops of the sterile diluent vials. Fill the syringe with the amount of air that is equal to the amount of sterile diluent you will remove from the first vial. Insert the needle through the rubber seal and slowly inject some of the air into the vial, allowing the fluid to flow into the syringe. Continue to push air gently into the vial until all of the fluid in the vial has flowed into the syringe. Remove any air that may be in the syringe as described above. Allow the pressure to equalize before you pull the needle out or you may lose fluid from the syringe. (If this occurs, the whole process needs to be repeated.) Withdraw the needle and replace the needle cap on the syringe. You may find it easier to hold the larger syringe in an upside down, vertical position while withdrawing the fluid in the vial.
To inject the sterile diluent into the cassette
Uncap the clamped cassette tube and carefully remove the needle from the syringe (discarding the needle in an appropriate manner). Attach the syringe to the cassette tubing. Unclamp the cassette tubing and carefully inject the solution into the cassette. When the syringe is empty, clamp the cassette tube near the syringe and disconnect the syringe. Replace the cap on the cassette tube. If more diluent is needed to fill the cassette, repeat steps 6 and 7 with an additional vial of diluent; however, after completing the transfer of all of the required diluent, clamp the tubing, but leave the syringe attached to the cassette tubing while you mix the solution. Gently turn the cassette upside down at least 10 times to thoroughly mix the reconstituted epoprostenol with the additional diluent.
To remove air from the cassette
To remove the air from inside the cassette, slowly turn the cassette until all of the small bubbles of air join to form one air pocket. Tilt the cassette gently so that the air pocket is in the corner where the tubing connects to the cassette. Unclamp the tube and pull back the plunger of the syringe until you see fluid fill the tubing. Clamp the tube near the connector and remove the syringe and replace the cap on the tubing. Label the cassette with the current time and date. Store the cassette in the refrigerator (preferably, on the top shelf to avoid spilling any food or drink on it) until it is time to use it. Make up a new cassette each day and use the cassette you refrigerated the day before so that you will always have a back-up cassette.
To use the pump
The instructions for the use of the pump may vary depending on the particular make and model of the pump. Your doctor or nurse will give detailed instructions on how to use and care for the particular pump and accessories that you will use for administering your medicine. These instructions should include how to change the pump battery, cassette, and tubing. Remember to change the gel packs every 12 hours or every 8 hours if the surrounding temperature approaches 86 °F.
Maintain sterile technique at all times. If you suspect that you have contaminated anything, throw away the accessories and begin again.
Dosing
The dose of this medicine will be different for different patients. Follow your doctor's orders or the directions on the label. The following information includes only the average doses of this medicine. If your dose is different, do not change it unless your doctor tells you to do so.
The amount of medicine that you take depends on the strength of the medicine. Also, the number of doses you take each day, the time allowed between doses, and the length of time you take the medicine depend on the medical problem for which you are using the medicine.
The amount of medicine that you take depends on the concentration of the reconstituted medicine and the rate at which the infusion pump delivers the medicine.
For injection dosage form:
For primary pulmonary hypertension and pulmonary hypertension secondary to scleroderma spectrum of disease:
Adults—Initially, 2 nanograms per kilogram (kg) (0.9 nanogram per pound) of body weight per minute. Your doctor may increase your dose as necessary.
Children—Use and dose must be determined by your doctor.
Missed Dose
Call your doctor or pharmacist for instructions.
Epoprostenol has to be administered by a continuous intravenous infusion and it must never be stopped suddenly.
Storage
Store the medicine in a closed container at room temperature, away from heat, moisture, and direct light. Keep from freezing.
Keep out of the reach of children.
Do not keep outdated medicine or medicine no longer needed.
Store the reconstituted injection in the refrigerator, away from direct light. However, keep the medicine from freezing. Any medicine that has been frozen should be thrown away. Reconstituted solutions should be kept either in the refrigerator or in a cold pouch, or a combination of the two, for no more than 48 hours. Do not expose reconstituted solution to temperatures higher than 25 °C (77 °F). If the reconstituted solution has particles in it or is discolored, it should be discarded.
Precautions While Using Flolan
It is important that your doctor check your progress at regular visits. This will allow your doctor to make sure the medicine is working properly and to change the dosage if needed.
Be sure to report any signs of infection at the catheter site to your doctor. Also, if you develop a sudden fever, contact your doctor as soon as possible.
Avoid the use of saunas, hot baths, or sunbathing, or other situations that may cause blood vessels to dilate, resulting in low blood pressure and increasing the possibility of dizziness, light-headedness, or fainting.
Do not suddenly stop using this medicine. Stopping suddenly may bring on symptoms of your condition and can be dangerous. Check with your doctor before stopping completely.
Your doctor may want you to carry a medical identification card stating that you are using this medicine.
Flolan Side Effects
Along with its needed effects, a medicine may cause some unwanted effects. Although not all of these side effects may occur, if they do occur they may need medical attention.
Check with your doctor immediately if any of the following side effects occur:
Signs and symptoms that can occur with initial dosage adjustments and/or dosage excess
Diarrhea
fast heartbeat
headache
light-headedness or fainting
nausea
redness of face or neck (flushing)
vomiting
Check with your doctor as soon as possible if any of the following side effects occur:
More common
Anxiety and/or nervousness
diarrhea
dizziness
flu or infection-like symptoms, such as chills, confusion, delirium, light-headedness or fainting, fast heartbeat, fever, and/or rapid, shallow breathing
headache
jaw pain (when chewing)
local infection at the catheter site
pain at injection site
pain in muscles or bones
redness of face (flushing)
unusual bleeding such as nosebleeds or bleeding gums or bruising
Less common
Altered or abnormal touch sensation or sensitivity
After you stop using this medicine, it may still produce some side effects that need attention. During this period of time, check with your doctor immediately if you notice the following side effects:
Difficult or labored breathing
dizziness
fainting
weakness
Other side effects not listed may also occur in some patients. If you notice any other effects, check with your healthcare professional.
FLOLAN for Injection 0.5mg(依前列醇钠注射剂)[/caption]
药店国别:
产地国家:美国
处 方 药:是
所属类别:0.5毫克/毫升/瓶
包装规格:0.5毫克/毫升/瓶
计价单位:瓶
生产厂家中文参考译名:GlaxoSmithKline LLC
生产厂家英文名:GlaxoSmithKline LLC
原产地英文商品名:FLOLAN 0.5 MG POWDER AND SOLVENT FOR SOLUTION FOR INFUSION
原产地英文药品名:EPOPROSTENOL SODIUM
中文参考商品译名:服疗能 粉剂和溶剂解输液 0.5毫克/毫升/瓶
中文参考药品译名:依前列醇钠
简介:
部份中文依前列醇钠处方资料(仅供参考)
药品英文名:Epoprostenol
药品别名:前列环素、依前列醇钠、环前列烯醇、环依前列烯醇、前列环素I2、PGX、Cycloprostin、PGI-2、Prostacyclin
药物剂型:注射剂(粉)(钠盐):500μg。临用时以pH 10.5的含甘氨酸的专用缓冲剂溶解。
药理作用:本品可通过增加血小板中的cAMP抑制血小板聚集,为已发现抗凝药中最强者。本品通过肺循环时不被代谢,可防止体外循环时血栓形成;较高剂量可使血小板凝块解聚,使皮肤出血时间延长2倍;可降低血小板的促凝作用,并减少其肝素中和因子的释放。本品可抑制ADP、胶原、花生四烯酸等诱导的血小板聚集和释放,且具有解聚作用。
药动学:参见:前列腺素E1
适应证:用于心肺分流术及炭血液灌注时保护血小板功能;也用于肾透析时代替肝素。用于冠状粥样硬化性心脏病不稳定型心绞痛、心肌梗死等。
禁忌证:有出血倾向者禁用。儿童、孕妇、哺乳妇女不宜使用。
注意事项:
1.在心肺分流术或血流灌注时不可代替肝素,仅在肾透析时代替肝素。若透析回路发生凝块,应停止透析。
2.超剂量使用可发生降压,应减量或停药。
不良反应:
1.高剂量时可见血压下降、心搏徐缓、面部潮红、头痛,以及胃痉挛痛、恶心、呕吐、腹部不适等。
2.对自发性或药物性出血者,应考虑引起出血并发症的可能。
用法用量:
1.静脉滴注:成人心肺分流术先每分钟连续静脉滴注10ng/kg;在分流术中每分钟静脉滴注20ng/kg,术毕即停注。
2.炭血灌注:先每分钟静脉滴注2~16ng/kg,灌注时每分钟静脉注射16ng/kg于碳柱的近侧管内。肾透析:透析前每分钟静脉滴注5ng/kg,透析时滴注于透析器的动脉入口。对老年人用剂量尚未验证是否需要修正。
药物相应作用:本品与抗凝剂、血管扩张药以及影响心血管反射的药物并用时有协同作用,需慎重。
专家点评:
本品可通过增加血小板中的cAMP抑制血小板聚集,为已发现的抗凝药中最强者。本品通过肺循环时不被代谢,可防止体外循环时血栓形成,较高剂量可使血小板凝块解聚,延长皮肤出血时间2倍,可降低血小板的促凝作用,并减少其肝素中和因子的释放。
FlolanGeneric Name: epoprostenol (Intravenous route)
e-poe-PROST-en-ol
Commonly used brand name(s):
In the U.S.
Flolan
Available Dosage Forms:
Powder for Solution
Therapeutic Class: Peripheral Vasodilator
Pharmacologic Class: Prostaglandin
Uses For Flolan
Epoprostenol belongs to a group of agents called prostaglandins. Prostaglandins occur naturally in the body and are involved in many biological functions. Epoprostenol is used to treat the symptoms of primary pulmonary hypertension, or the high blood pressure that occurs in the main artery that carries blood from the right side of the heart (the ventricle) to the lungs. When the smaller blood vessels in the lungs become more resistant to blood flow, the right ventricle must work harder to pump enough blood through the lungs. Epoprostenol works by relaxing blood vessels and increasing the supply of blood to the lungs, reducing the workload of the heart.
This medicine is available only with your doctor's prescription.
Before Using Flolan
In deciding to use a medicine, the risks of taking the medicine must be weighed against the good it will do. This is a decision you and your doctor will make. For this medicine, the following should be considered:
Allergies
Tell your doctor if you have ever had any unusual or allergic reaction to this medicine or any other medicines. Also tell your health care professional if you have any other types of allergies, such as to foods, dyes, preservatives, or animals. For non-prescription products, read the label or package ingredients carefully.
Pediatric
Although there is no specific information comparing use of this medicine in children with use in other age groups, this medicine is not expected to cause different side effects or problems in children than it does in adults.
Geriatric
Many medicines have not been studied specifically in older people. Therefore, it may not be known whether they work exactly the same way they do in younger adults or if they cause different side effects or problems in older people. There is no specific information comparing use of epoprostenol in the elderly with use in other age groups.
Pregnancy
Pregnancy Category Explanation
All Trimesters B Animal studies have revealed no evidence of harm to the fetus, however, there are no adequate studies in pregnant women OR animal studies have shown an adverse effect, but adequate studies in pregnant women have failed to demonstrate a risk to the fetus.
Breast Feeding
There are no adequate studies in women for determining infant risk when using this medication during breastfeeding. Weigh the potential benefits against the potential risks before taking this medication while breastfeeding.
Interactions with Medicines
Using this medicine with any of the following medicines is usually not recommended, but may be required in some cases. If both medicines are prescribed together, your doctor may change the dose or how often you use one or both of the medicines.
Desvenlafaxine
Duloxetine
Milnacipran
Venlafaxine
Using this medicine with any of the following medicines may cause an increased risk of certain side effects, but using both drugs may be the best treatment for you. If both medicines are prescribed together, your doctor may change the dose or how often you use one or both of the medicines.
Digoxin
Interactions with Food/Tobacco/Alcohol
Certain medicines should not be used at or around the time of eating food or eating certain types of food since interactions may occur. Using alcohol or tobacco with certain medicines may also cause interactions to occur. Discuss with your healthcare professional the use of your medicine with food, alcohol, or tobacco.
Other Medical Problems
The presence of other medical problems may affect the use of this medicine. Make sure you tell your doctor if you have any other medical problems, especially:
Heart disease or
Lung disease—Epoprostenol may make these conditions worse
Proper Use of Flolan
Your doctor or nurse will teach you how to prepare the medicine and use the pump for administering the medicine. Epoprostenol must be administered continuously by a portable pump that is operated by a small computer. The medicine will be delivered directly to the heart through a catheter that will be inserted into a vein in the chest.
Epoprostenol should be reconstituted only with the sterile diluent that is supplied with this medicine. The reconstituted medicine should not be mixed with other solutions or medicines. Use the following procedure for reconstituting your daily supply:
Clear an area to work in and clean the area with alcohol. Gather your supplies. Wash your hands thoroughly with soap and water and then open all packages. Remove the vial cap from the vial containing the sterile diluent, clean the tops of the vials with alcohol swabs, and let the vial tops dry before proceeding.
To withdraw the sterile diluent
If not already attached, attach a needle to the syringe. Gently pull the plunger out slightly and push it back to break the syringe seal. Draw air into the syringe that is about equal to the amount of sterile diluent you've been instructed to withdraw from the vial. Insert the needle at an angle, completely through the rubber seal of the vial. Turn the vial and syringe upside down (the syringe-vial unit is now vertical) and carefully press the plunger, injecting some or all of the air into the vial. Then aim the tip of the needle into the fluid and carefully pull the plunger slowly back to withdraw the diluent and/or allow the pressure to fill the syringe with the diluent. Continue pushing the remaining air into the vial, allowing the liquid to enter the syringe until the prescribed amount of diluent has been drawn into the syringe. Without withdrawing the needle, tap the syringe gently so that any air bubbles trapped in the syringe rise toward the top of the syringe. If air bubbles appear, depress the plunger gently to force the air bubbles out (into the vial) and then withdraw enough additional diluent to restore the needed volume in the syringe. (Holding the syringe-vial as a unit in a vertical position and keeping the needle tip in the fluid while withdrawing the diluent may help minimize the amount of air drawn into the syringe.) Once the required volume has been drawn into the syringe, let the syringe-vial pressure equalize and slowly withdraw the needle from the vial.
To reconstitute the epoprostenol
Insert the same needle through the rubber seal of the vial of epoprostenol and inject the sterile diluent gently onto the side of the vial. The flow of the sterile diluent should be directed toward the side of the vial and injected slowly in order to prevent the medicine from foaming. Once the pressure has equalized, withdraw the needle from the vial. Gently swirl the vial to mix the epoprostenol. Turn the vial upside down to catch any undissolved powder near the top of the vial. Never shake the vials. Repeat this process if you need to mix more than one vial of epoprostenol.
To draw out the reconstituted epoprostenol
Wipe the top of the reconstituted epoprostenol vial with an alcohol swab and let it dry. Change the needle on the syringe and then gently pull back the syringe plunger and fill the syringe with the amount of air that is equal to the amount of reconstituted epoprostenol you have been instructed to withdraw. Insert the needle through the seal of the vial and inject the air into the vial. Be sure to keep the needle tip below the fluid line and then pull the plunger back gently to withdraw the reconstituted epoprostenol into the syringe. Remove any air that may be trapped in the syringe as described above. Withdraw the needle and replace the needle cap on the syringe.
To inject the reconstituted epoprostenol into the cassette
Remove the end cap from the cassette tubing. Carefully remove the needle from the syringe (be sure to discard the needle in an appropriate manner) and attach the syringe to the cassette tubing. Hold the cassette in one hand and push the plunger to inject the reconstituted solution into the cassette (alternatively, you may find it useful to use a tabletop or other solid structure to steady the plunger while pushing down on the syringe to inject the solution). When the syringe is empty, clamp the cassette tubing near the syringe. Disconnect the syringe and replace the cassette tubing end cap.
To inject the remaining diluent into the partially filled cassette
Using a 60 mL syringe, attach a new needle to the syringe and follow the above procedures for breaking the syringe seal and wiping the tops of the sterile diluent vials. Fill the syringe with the amount of air that is equal to the amount of sterile diluent you will remove from the first vial. Insert the needle through the rubber seal and slowly inject some of the air into the vial, allowing the fluid to flow into the syringe. Continue to push air gently into the vial until all of the fluid in the vial has flowed into the syringe. Remove any air that may be in the syringe as described above. Allow the pressure to equalize before you pull the needle out or you may lose fluid from the syringe. (If this occurs, the whole process needs to be repeated.) Withdraw the needle and replace the needle cap on the syringe. You may find it easier to hold the larger syringe in an upside down, vertical position while withdrawing the fluid in the vial.
To inject the sterile diluent into the cassette
Uncap the clamped cassette tube and carefully remove the needle from the syringe (discarding the needle in an appropriate manner). Attach the syringe to the cassette tubing. Unclamp the cassette tubing and carefully inject the solution into the cassette. When the syringe is empty, clamp the cassette tube near the syringe and disconnect the syringe. Replace the cap on the cassette tube. If more diluent is needed to fill the cassette, repeat steps 6 and 7 with an additional vial of diluent; however, after completing the transfer of all of the required diluent, clamp the tubing, but leave the syringe attached to the cassette tubing while you mix the solution. Gently turn the cassette upside down at least 10 times to thoroughly mix the reconstituted epoprostenol with the additional diluent.
To remove air from the cassette
To remove the air from inside the cassette, slowly turn the cassette until all of the small bubbles of air join to form one air pocket. Tilt the cassette gently so that the air pocket is in the corner where the tubing connects to the cassette. Unclamp the tube and pull back the plunger of the syringe until you see fluid fill the tubing. Clamp the tube near the connector and remove the syringe and replace the cap on the tubing. Label the cassette with the current time and date. Store the cassette in the refrigerator (preferably, on the top shelf to avoid spilling any food or drink on it) until it is time to use it. Make up a new cassette each day and use the cassette you refrigerated the day before so that you will always have a back-up cassette.
To use the pump
The instructions for the use of the pump may vary depending on the particular make and model of the pump. Your doctor or nurse will give detailed instructions on how to use and care for the particular pump and accessories that you will use for administering your medicine. These instructions should include how to change the pump battery, cassette, and tubing. Remember to change the gel packs every 12 hours or every 8 hours if the surrounding temperature approaches 86 °F.
Maintain sterile technique at all times. If you suspect that you have contaminated anything, throw away the accessories and begin again.
Dosing
The dose of this medicine will be different for different patients. Follow your doctor's orders or the directions on the label. The following information includes only the average doses of this medicine. If your dose is different, do not change it unless your doctor tells you to do so.
The amount of medicine that you take depends on the strength of the medicine. Also, the number of doses you take each day, the time allowed between doses, and the length of time you take the medicine depend on the medical problem for which you are using the medicine.
The amount of medicine that you take depends on the concentration of the reconstituted medicine and the rate at which the infusion pump delivers the medicine.
For injection dosage form:
For primary pulmonary hypertension and pulmonary hypertension secondary to scleroderma spectrum of disease:
Adults—Initially, 2 nanograms per kilogram (kg) (0.9 nanogram per pound) of body weight per minute. Your doctor may increase your dose as necessary.
Children—Use and dose must be determined by your doctor.
Missed Dose
Call your doctor or pharmacist for instructions.
Epoprostenol has to be administered by a continuous intravenous infusion and it must never be stopped suddenly.
Storage
Store the medicine in a closed container at room temperature, away from heat, moisture, and direct light. Keep from freezing.
Keep out of the reach of children.
Do not keep outdated medicine or medicine no longer needed.
Store the reconstituted injection in the refrigerator, away from direct light. However, keep the medicine from freezing. Any medicine that has been frozen should be thrown away. Reconstituted solutions should be kept either in the refrigerator or in a cold pouch, or a combination of the two, for no more than 48 hours. Do not expose reconstituted solution to temperatures higher than 25 °C (77 °F). If the reconstituted solution has particles in it or is discolored, it should be discarded.
Precautions While Using Flolan
It is important that your doctor check your progress at regular visits. This will allow your doctor to make sure the medicine is working properly and to change the dosage if needed.
Be sure to report any signs of infection at the catheter site to your doctor. Also, if you develop a sudden fever, contact your doctor as soon as possible.
Avoid the use of saunas, hot baths, or sunbathing, or other situations that may cause blood vessels to dilate, resulting in low blood pressure and increasing the possibility of dizziness, light-headedness, or fainting.
Do not suddenly stop using this medicine. Stopping suddenly may bring on symptoms of your condition and can be dangerous. Check with your doctor before stopping completely.
Your doctor may want you to carry a medical identification card stating that you are using this medicine.
Flolan Side Effects
Along with its needed effects, a medicine may cause some unwanted effects. Although not all of these side effects may occur, if they do occur they may need medical attention.
Check with your doctor immediately if any of the following side effects occur:
Signs and symptoms that can occur with initial dosage adjustments and/or dosage excess
Diarrhea
fast heartbeat
headache
light-headedness or fainting
nausea
redness of face or neck (flushing)
vomiting
Check with your doctor as soon as possible if any of the following side effects occur:
More common
Anxiety and/or nervousness
diarrhea
dizziness
flu or infection-like symptoms, such as chills, confusion, delirium, light-headedness or fainting, fast heartbeat, fever, and/or rapid, shallow breathing
headache
jaw pain (when chewing)
local infection at the catheter site
pain at injection site
pain in muscles or bones
redness of face (flushing)
unusual bleeding such as nosebleeds or bleeding gums or bruising
Less common
Altered or abnormal touch sensation or sensitivity
After you stop using this medicine, it may still produce some side effects that need attention. During this period of time, check with your doctor immediately if you notice the following side effects:
Difficult or labored breathing
dizziness
fainting
weakness
Other side effects not listed may also occur in some patients. If you notice any other effects, check with your healthcare professional.
 FLOLAN for Injection 0.5mg(依前列醇钠注射剂)[/caption]
药店国别:
产地国家:美国
处 方 药:是
所属类别:0.5毫克/毫升/瓶
包装规格:0.5毫克/毫升/瓶
计价单位:瓶
生产厂家中文参考译名:GlaxoSmithKline LLC
生产厂家英文名:GlaxoSmithKline LLC
原产地英文商品名:FLOLAN 0.5 MG POWDER AND SOLVENT FOR SOLUTION FOR INFUSION
原产地英文药品名:EPOPROSTENOL SODIUM
中文参考商品译名:服疗能 粉剂和溶剂解输液 0.5毫克/毫升/瓶
中文参考药品译名:依前列醇钠
简介:
部份中文依前列醇钠处方资料(仅供参考)
药品英文名:Epoprostenol
药品别名:前列环素、依前列醇钠、环前列烯醇、环依前列烯醇、前列环素I2、PGX、Cycloprostin、PGI-2、Prostacyclin
药物剂型:注射剂(粉)(钠盐):500μg。临用时以pH 10.5的含甘氨酸的专用缓冲剂溶解。
药理作用:本品可通过增加血小板中的cAMP抑制血小板聚集,为已发现抗凝药中最强者。本品通过肺循环时不被代谢,可防止体外循环时血栓形成;较高剂量可使血小板凝块解聚,使皮肤出血时间延长2倍;可降低血小板的促凝作用,并减少其肝素中和因子的释放。本品可抑制ADP、胶原、花生四烯酸等诱导的血小板聚集和释放,且具有解聚作用。
药动学:参见:前列腺素E1
适应证:用于心肺分流术及炭血液灌注时保护血小板功能;也用于肾透析时代替肝素。用于冠状粥样硬化性心脏病不稳定型心绞痛、心肌梗死等。
禁忌证:有出血倾向者禁用。儿童、孕妇、哺乳妇女不宜使用。
注意事项:
1.在心肺分流术或血流灌注时不可代替肝素,仅在肾透析时代替肝素。若透析回路发生凝块,应停止透析。
2.超剂量使用可发生降压,应减量或停药。
不良反应:
1.高剂量时可见血压下降、心搏徐缓、面部潮红、头痛,以及胃痉挛痛、恶心、呕吐、腹部不适等。
2.对自发性或药物性出血者,应考虑引起出血并发症的可能。
用法用量:
1.静脉滴注:成人心肺分流术先每分钟连续静脉滴注10ng/kg;在分流术中每分钟静脉滴注20ng/kg,术毕即停注。
2.炭血灌注:先每分钟静脉滴注2~16ng/kg,灌注时每分钟静脉注射16ng/kg于碳柱的近侧管内。肾透析:透析前每分钟静脉滴注5ng/kg,透析时滴注于透析器的动脉入口。对老年人用剂量尚未验证是否需要修正。
药物相应作用:本品与抗凝剂、血管扩张药以及影响心血管反射的药物并用时有协同作用,需慎重。
专家点评:
本品可通过增加血小板中的cAMP抑制血小板聚集,为已发现的抗凝药中最强者。本品通过肺循环时不被代谢,可防止体外循环时血栓形成,较高剂量可使血小板凝块解聚,延长皮肤出血时间2倍,可降低血小板的促凝作用,并减少其肝素中和因子的释放。
FlolanGeneric Name: epoprostenol (Intravenous route)
e-poe-PROST-en-ol
Commonly used brand name(s):
In the U.S.
Flolan
Available Dosage Forms:
Powder for Solution
Therapeutic Class: Peripheral Vasodilator
Pharmacologic Class: Prostaglandin
Uses For Flolan
Epoprostenol belongs to a group of agents called prostaglandins. Prostaglandins occur naturally in the body and are involved in many biological functions. Epoprostenol is used to treat the symptoms of primary pulmonary hypertension, or the high blood pressure that occurs in the main artery that carries blood from the right side of the heart (the ventricle) to the lungs. When the smaller blood vessels in the lungs become more resistant to blood flow, the right ventricle must work harder to pump enough blood through the lungs. Epoprostenol works by relaxing blood vessels and increasing the supply of blood to the lungs, reducing the workload of the heart.
This medicine is available only with your doctor's prescription.
Before Using Flolan
In deciding to use a medicine, the risks of taking the medicine must be weighed against the good it will do. This is a decision you and your doctor will make. For this medicine, the following should be considered:
Allergies
Tell your doctor if you have ever had any unusual or allergic reaction to this medicine or any other medicines. Also tell your health care professional if you have any other types of allergies, such as to foods, dyes, preservatives, or animals. For non-prescription products, read the label or package ingredients carefully.
Pediatric
Although there is no specific information comparing use of this medicine in children with use in other age groups, this medicine is not expected to cause different side effects or problems in children than it does in adults.
Geriatric
Many medicines have not been studied specifically in older people. Therefore, it may not be known whether they work exactly the same way they do in younger adults or if they cause different side effects or problems in older people. There is no specific information comparing use of epoprostenol in the elderly with use in other age groups.
Pregnancy
Pregnancy Category Explanation
All Trimesters B Animal studies have revealed no evidence of harm to the fetus, however, there are no adequate studies in pregnant women OR animal studies have shown an adverse effect, but adequate studies in pregnant women have failed to demonstrate a risk to the fetus.
Breast Feeding
There are no adequate studies in women for determining infant risk when using this medication during breastfeeding. Weigh the potential benefits against the potential risks before taking this medication while breastfeeding.
Interactions with Medicines
Using this medicine with any of the following medicines is usually not recommended, but may be required in some cases. If both medicines are prescribed together, your doctor may change the dose or how often you use one or both of the medicines.
Desvenlafaxine
Duloxetine
Milnacipran
Venlafaxine
Using this medicine with any of the following medicines may cause an increased risk of certain side effects, but using both drugs may be the best treatment for you. If both medicines are prescribed together, your doctor may change the dose or how often you use one or both of the medicines.
Digoxin
Interactions with Food/Tobacco/Alcohol
Certain medicines should not be used at or around the time of eating food or eating certain types of food since interactions may occur. Using alcohol or tobacco with certain medicines may also cause interactions to occur. Discuss with your healthcare professional the use of your medicine with food, alcohol, or tobacco.
Other Medical Problems
The presence of other medical problems may affect the use of this medicine. Make sure you tell your doctor if you have any other medical problems, especially:
Heart disease or
Lung disease—Epoprostenol may make these conditions worse
Proper Use of Flolan
Your doctor or nurse will teach you how to prepare the medicine and use the pump for administering the medicine. Epoprostenol must be administered continuously by a portable pump that is operated by a small computer. The medicine will be delivered directly to the heart through a catheter that will be inserted into a vein in the chest.
Epoprostenol should be reconstituted only with the sterile diluent that is supplied with this medicine. The reconstituted medicine should not be mixed with other solutions or medicines. Use the following procedure for reconstituting your daily supply:
Clear an area to work in and clean the area with alcohol. Gather your supplies. Wash your hands thoroughly with soap and water and then open all packages. Remove the vial cap from the vial containing the sterile diluent, clean the tops of the vials with alcohol swabs, and let the vial tops dry before proceeding.
To withdraw the sterile diluent
If not already attached, attach a needle to the syringe. Gently pull the plunger out slightly and push it back to break the syringe seal. Draw air into the syringe that is about equal to the amount of sterile diluent you've been instructed to withdraw from the vial. Insert the needle at an angle, completely through the rubber seal of the vial. Turn the vial and syringe upside down (the syringe-vial unit is now vertical) and carefully press the plunger, injecting some or all of the air into the vial. Then aim the tip of the needle into the fluid and carefully pull the plunger slowly back to withdraw the diluent and/or allow the pressure to fill the syringe with the diluent. Continue pushing the remaining air into the vial, allowing the liquid to enter the syringe until the prescribed amount of diluent has been drawn into the syringe. Without withdrawing the needle, tap the syringe gently so that any air bubbles trapped in the syringe rise toward the top of the syringe. If air bubbles appear, depress the plunger gently to force the air bubbles out (into the vial) and then withdraw enough additional diluent to restore the needed volume in the syringe. (Holding the syringe-vial as a unit in a vertical position and keeping the needle tip in the fluid while withdrawing the diluent may help minimize the amount of air drawn into the syringe.) Once the required volume has been drawn into the syringe, let the syringe-vial pressure equalize and slowly withdraw the needle from the vial.
To reconstitute the epoprostenol
Insert the same needle through the rubber seal of the vial of epoprostenol and inject the sterile diluent gently onto the side of the vial. The flow of the sterile diluent should be directed toward the side of the vial and injected slowly in order to prevent the medicine from foaming. Once the pressure has equalized, withdraw the needle from the vial. Gently swirl the vial to mix the epoprostenol. Turn the vial upside down to catch any undissolved powder near the top of the vial. Never shake the vials. Repeat this process if you need to mix more than one vial of epoprostenol.
To draw out the reconstituted epoprostenol
Wipe the top of the reconstituted epoprostenol vial with an alcohol swab and let it dry. Change the needle on the syringe and then gently pull back the syringe plunger and fill the syringe with the amount of air that is equal to the amount of reconstituted epoprostenol you have been instructed to withdraw. Insert the needle through the seal of the vial and inject the air into the vial. Be sure to keep the needle tip below the fluid line and then pull the plunger back gently to withdraw the reconstituted epoprostenol into the syringe. Remove any air that may be trapped in the syringe as described above. Withdraw the needle and replace the needle cap on the syringe.
To inject the reconstituted epoprostenol into the cassette
Remove the end cap from the cassette tubing. Carefully remove the needle from the syringe (be sure to discard the needle in an appropriate manner) and attach the syringe to the cassette tubing. Hold the cassette in one hand and push the plunger to inject the reconstituted solution into the cassette (alternatively, you may find it useful to use a tabletop or other solid structure to steady the plunger while pushing down on the syringe to inject the solution). When the syringe is empty, clamp the cassette tubing near the syringe. Disconnect the syringe and replace the cassette tubing end cap.
To inject the remaining diluent into the partially filled cassette
Using a 60 mL syringe, attach a new needle to the syringe and follow the above procedures for breaking the syringe seal and wiping the tops of the sterile diluent vials. Fill the syringe with the amount of air that is equal to the amount of sterile diluent you will remove from the first vial. Insert the needle through the rubber seal and slowly inject some of the air into the vial, allowing the fluid to flow into the syringe. Continue to push air gently into the vial until all of the fluid in the vial has flowed into the syringe. Remove any air that may be in the syringe as described above. Allow the pressure to equalize before you pull the needle out or you may lose fluid from the syringe. (If this occurs, the whole process needs to be repeated.) Withdraw the needle and replace the needle cap on the syringe. You may find it easier to hold the larger syringe in an upside down, vertical position while withdrawing the fluid in the vial.
To inject the sterile diluent into the cassette
Uncap the clamped cassette tube and carefully remove the needle from the syringe (discarding the needle in an appropriate manner). Attach the syringe to the cassette tubing. Unclamp the cassette tubing and carefully inject the solution into the cassette. When the syringe is empty, clamp the cassette tube near the syringe and disconnect the syringe. Replace the cap on the cassette tube. If more diluent is needed to fill the cassette, repeat steps 6 and 7 with an additional vial of diluent; however, after completing the transfer of all of the required diluent, clamp the tubing, but leave the syringe attached to the cassette tubing while you mix the solution. Gently turn the cassette upside down at least 10 times to thoroughly mix the reconstituted epoprostenol with the additional diluent.
To remove air from the cassette
To remove the air from inside the cassette, slowly turn the cassette until all of the small bubbles of air join to form one air pocket. Tilt the cassette gently so that the air pocket is in the corner where the tubing connects to the cassette. Unclamp the tube and pull back the plunger of the syringe until you see fluid fill the tubing. Clamp the tube near the connector and remove the syringe and replace the cap on the tubing. Label the cassette with the current time and date. Store the cassette in the refrigerator (preferably, on the top shelf to avoid spilling any food or drink on it) until it is time to use it. Make up a new cassette each day and use the cassette you refrigerated the day before so that you will always have a back-up cassette.
To use the pump
The instructions for the use of the pump may vary depending on the particular make and model of the pump. Your doctor or nurse will give detailed instructions on how to use and care for the particular pump and accessories that you will use for administering your medicine. These instructions should include how to change the pump battery, cassette, and tubing. Remember to change the gel packs every 12 hours or every 8 hours if the surrounding temperature approaches 86 °F.
Maintain sterile technique at all times. If you suspect that you have contaminated anything, throw away the accessories and begin again.
Dosing
The dose of this medicine will be different for different patients. Follow your doctor's orders or the directions on the label. The following information includes only the average doses of this medicine. If your dose is different, do not change it unless your doctor tells you to do so.
The amount of medicine that you take depends on the strength of the medicine. Also, the number of doses you take each day, the time allowed between doses, and the length of time you take the medicine depend on the medical problem for which you are using the medicine.
The amount of medicine that you take depends on the concentration of the reconstituted medicine and the rate at which the infusion pump delivers the medicine.
For injection dosage form:
For primary pulmonary hypertension and pulmonary hypertension secondary to scleroderma spectrum of disease:
Adults—Initially, 2 nanograms per kilogram (kg) (0.9 nanogram per pound) of body weight per minute. Your doctor may increase your dose as necessary.
Children—Use and dose must be determined by your doctor.
Missed Dose
Call your doctor or pharmacist for instructions.
Epoprostenol has to be administered by a continuous intravenous infusion and it must never be stopped suddenly.
Storage
Store the medicine in a closed container at room temperature, away from heat, moisture, and direct light. Keep from freezing.
Keep out of the reach of children.
Do not keep outdated medicine or medicine no longer needed.
Store the reconstituted injection in the refrigerator, away from direct light. However, keep the medicine from freezing. Any medicine that has been frozen should be thrown away. Reconstituted solutions should be kept either in the refrigerator or in a cold pouch, or a combination of the two, for no more than 48 hours. Do not expose reconstituted solution to temperatures higher than 25 °C (77 °F). If the reconstituted solution has particles in it or is discolored, it should be discarded.
Precautions While Using Flolan
It is important that your doctor check your progress at regular visits. This will allow your doctor to make sure the medicine is working properly and to change the dosage if needed.
Be sure to report any signs of infection at the catheter site to your doctor. Also, if you develop a sudden fever, contact your doctor as soon as possible.
Avoid the use of saunas, hot baths, or sunbathing, or other situations that may cause blood vessels to dilate, resulting in low blood pressure and increasing the possibility of dizziness, light-headedness, or fainting.
Do not suddenly stop using this medicine. Stopping suddenly may bring on symptoms of your condition and can be dangerous. Check with your doctor before stopping completely.
Your doctor may want you to carry a medical identification card stating that you are using this medicine.
Flolan Side Effects
Along with its needed effects, a medicine may cause some unwanted effects. Although not all of these side effects may occur, if they do occur they may need medical attention.
Check with your doctor immediately if any of the following side effects occur:
Signs and symptoms that can occur with initial dosage adjustments and/or dosage excess
Diarrhea
fast heartbeat
headache
light-headedness or fainting
nausea
redness of face or neck (flushing)
vomiting
Check with your doctor as soon as possible if any of the following side effects occur:
More common
Anxiety and/or nervousness
diarrhea
dizziness
flu or infection-like symptoms, such as chills, confusion, delirium, light-headedness or fainting, fast heartbeat, fever, and/or rapid, shallow breathing
headache
jaw pain (when chewing)
local infection at the catheter site
pain at injection site
pain in muscles or bones
redness of face (flushing)
unusual bleeding such as nosebleeds or bleeding gums or bruising
Less common
Altered or abnormal touch sensation or sensitivity
After you stop using this medicine, it may still produce some side effects that need attention. During this period of time, check with your doctor immediately if you notice the following side effects:
Difficult or labored breathing
dizziness
fainting
weakness
Other side effects not listed may also occur in some patients. If you notice any other effects, check with your healthcare professional.
FLOLAN for Injection 0.5mg(依前列醇钠注射剂)[/caption]
药店国别:
产地国家:美国
处 方 药:是
所属类别:0.5毫克/毫升/瓶
包装规格:0.5毫克/毫升/瓶
计价单位:瓶
生产厂家中文参考译名:GlaxoSmithKline LLC
生产厂家英文名:GlaxoSmithKline LLC
原产地英文商品名:FLOLAN 0.5 MG POWDER AND SOLVENT FOR SOLUTION FOR INFUSION
原产地英文药品名:EPOPROSTENOL SODIUM
中文参考商品译名:服疗能 粉剂和溶剂解输液 0.5毫克/毫升/瓶
中文参考药品译名:依前列醇钠
简介:
部份中文依前列醇钠处方资料(仅供参考)
药品英文名:Epoprostenol
药品别名:前列环素、依前列醇钠、环前列烯醇、环依前列烯醇、前列环素I2、PGX、Cycloprostin、PGI-2、Prostacyclin
药物剂型:注射剂(粉)(钠盐):500μg。临用时以pH 10.5的含甘氨酸的专用缓冲剂溶解。
药理作用:本品可通过增加血小板中的cAMP抑制血小板聚集,为已发现抗凝药中最强者。本品通过肺循环时不被代谢,可防止体外循环时血栓形成;较高剂量可使血小板凝块解聚,使皮肤出血时间延长2倍;可降低血小板的促凝作用,并减少其肝素中和因子的释放。本品可抑制ADP、胶原、花生四烯酸等诱导的血小板聚集和释放,且具有解聚作用。
药动学:参见:前列腺素E1
适应证:用于心肺分流术及炭血液灌注时保护血小板功能;也用于肾透析时代替肝素。用于冠状粥样硬化性心脏病不稳定型心绞痛、心肌梗死等。
禁忌证:有出血倾向者禁用。儿童、孕妇、哺乳妇女不宜使用。
注意事项:
1.在心肺分流术或血流灌注时不可代替肝素,仅在肾透析时代替肝素。若透析回路发生凝块,应停止透析。
2.超剂量使用可发生降压,应减量或停药。
不良反应:
1.高剂量时可见血压下降、心搏徐缓、面部潮红、头痛,以及胃痉挛痛、恶心、呕吐、腹部不适等。
2.对自发性或药物性出血者,应考虑引起出血并发症的可能。
用法用量:
1.静脉滴注:成人心肺分流术先每分钟连续静脉滴注10ng/kg;在分流术中每分钟静脉滴注20ng/kg,术毕即停注。
2.炭血灌注:先每分钟静脉滴注2~16ng/kg,灌注时每分钟静脉注射16ng/kg于碳柱的近侧管内。肾透析:透析前每分钟静脉滴注5ng/kg,透析时滴注于透析器的动脉入口。对老年人用剂量尚未验证是否需要修正。
药物相应作用:本品与抗凝剂、血管扩张药以及影响心血管反射的药物并用时有协同作用,需慎重。
专家点评:
本品可通过增加血小板中的cAMP抑制血小板聚集,为已发现的抗凝药中最强者。本品通过肺循环时不被代谢,可防止体外循环时血栓形成,较高剂量可使血小板凝块解聚,延长皮肤出血时间2倍,可降低血小板的促凝作用,并减少其肝素中和因子的释放。
FlolanGeneric Name: epoprostenol (Intravenous route)
e-poe-PROST-en-ol
Commonly used brand name(s):
In the U.S.
Flolan
Available Dosage Forms:
Powder for Solution
Therapeutic Class: Peripheral Vasodilator
Pharmacologic Class: Prostaglandin
Uses For Flolan
Epoprostenol belongs to a group of agents called prostaglandins. Prostaglandins occur naturally in the body and are involved in many biological functions. Epoprostenol is used to treat the symptoms of primary pulmonary hypertension, or the high blood pressure that occurs in the main artery that carries blood from the right side of the heart (the ventricle) to the lungs. When the smaller blood vessels in the lungs become more resistant to blood flow, the right ventricle must work harder to pump enough blood through the lungs. Epoprostenol works by relaxing blood vessels and increasing the supply of blood to the lungs, reducing the workload of the heart.
This medicine is available only with your doctor's prescription.
Before Using Flolan
In deciding to use a medicine, the risks of taking the medicine must be weighed against the good it will do. This is a decision you and your doctor will make. For this medicine, the following should be considered:
Allergies
Tell your doctor if you have ever had any unusual or allergic reaction to this medicine or any other medicines. Also tell your health care professional if you have any other types of allergies, such as to foods, dyes, preservatives, or animals. For non-prescription products, read the label or package ingredients carefully.
Pediatric
Although there is no specific information comparing use of this medicine in children with use in other age groups, this medicine is not expected to cause different side effects or problems in children than it does in adults.
Geriatric
Many medicines have not been studied specifically in older people. Therefore, it may not be known whether they work exactly the same way they do in younger adults or if they cause different side effects or problems in older people. There is no specific information comparing use of epoprostenol in the elderly with use in other age groups.
Pregnancy
Pregnancy Category Explanation
All Trimesters B Animal studies have revealed no evidence of harm to the fetus, however, there are no adequate studies in pregnant women OR animal studies have shown an adverse effect, but adequate studies in pregnant women have failed to demonstrate a risk to the fetus.
Breast Feeding
There are no adequate studies in women for determining infant risk when using this medication during breastfeeding. Weigh the potential benefits against the potential risks before taking this medication while breastfeeding.
Interactions with Medicines
Using this medicine with any of the following medicines is usually not recommended, but may be required in some cases. If both medicines are prescribed together, your doctor may change the dose or how often you use one or both of the medicines.
Desvenlafaxine
Duloxetine
Milnacipran
Venlafaxine
Using this medicine with any of the following medicines may cause an increased risk of certain side effects, but using both drugs may be the best treatment for you. If both medicines are prescribed together, your doctor may change the dose or how often you use one or both of the medicines.
Digoxin
Interactions with Food/Tobacco/Alcohol
Certain medicines should not be used at or around the time of eating food or eating certain types of food since interactions may occur. Using alcohol or tobacco with certain medicines may also cause interactions to occur. Discuss with your healthcare professional the use of your medicine with food, alcohol, or tobacco.
Other Medical Problems
The presence of other medical problems may affect the use of this medicine. Make sure you tell your doctor if you have any other medical problems, especially:
Heart disease or
Lung disease—Epoprostenol may make these conditions worse
Proper Use of Flolan
Your doctor or nurse will teach you how to prepare the medicine and use the pump for administering the medicine. Epoprostenol must be administered continuously by a portable pump that is operated by a small computer. The medicine will be delivered directly to the heart through a catheter that will be inserted into a vein in the chest.
Epoprostenol should be reconstituted only with the sterile diluent that is supplied with this medicine. The reconstituted medicine should not be mixed with other solutions or medicines. Use the following procedure for reconstituting your daily supply:
Clear an area to work in and clean the area with alcohol. Gather your supplies. Wash your hands thoroughly with soap and water and then open all packages. Remove the vial cap from the vial containing the sterile diluent, clean the tops of the vials with alcohol swabs, and let the vial tops dry before proceeding.
To withdraw the sterile diluent
If not already attached, attach a needle to the syringe. Gently pull the plunger out slightly and push it back to break the syringe seal. Draw air into the syringe that is about equal to the amount of sterile diluent you've been instructed to withdraw from the vial. Insert the needle at an angle, completely through the rubber seal of the vial. Turn the vial and syringe upside down (the syringe-vial unit is now vertical) and carefully press the plunger, injecting some or all of the air into the vial. Then aim the tip of the needle into the fluid and carefully pull the plunger slowly back to withdraw the diluent and/or allow the pressure to fill the syringe with the diluent. Continue pushing the remaining air into the vial, allowing the liquid to enter the syringe until the prescribed amount of diluent has been drawn into the syringe. Without withdrawing the needle, tap the syringe gently so that any air bubbles trapped in the syringe rise toward the top of the syringe. If air bubbles appear, depress the plunger gently to force the air bubbles out (into the vial) and then withdraw enough additional diluent to restore the needed volume in the syringe. (Holding the syringe-vial as a unit in a vertical position and keeping the needle tip in the fluid while withdrawing the diluent may help minimize the amount of air drawn into the syringe.) Once the required volume has been drawn into the syringe, let the syringe-vial pressure equalize and slowly withdraw the needle from the vial.
To reconstitute the epoprostenol
Insert the same needle through the rubber seal of the vial of epoprostenol and inject the sterile diluent gently onto the side of the vial. The flow of the sterile diluent should be directed toward the side of the vial and injected slowly in order to prevent the medicine from foaming. Once the pressure has equalized, withdraw the needle from the vial. Gently swirl the vial to mix the epoprostenol. Turn the vial upside down to catch any undissolved powder near the top of the vial. Never shake the vials. Repeat this process if you need to mix more than one vial of epoprostenol.
To draw out the reconstituted epoprostenol
Wipe the top of the reconstituted epoprostenol vial with an alcohol swab and let it dry. Change the needle on the syringe and then gently pull back the syringe plunger and fill the syringe with the amount of air that is equal to the amount of reconstituted epoprostenol you have been instructed to withdraw. Insert the needle through the seal of the vial and inject the air into the vial. Be sure to keep the needle tip below the fluid line and then pull the plunger back gently to withdraw the reconstituted epoprostenol into the syringe. Remove any air that may be trapped in the syringe as described above. Withdraw the needle and replace the needle cap on the syringe.
To inject the reconstituted epoprostenol into the cassette
Remove the end cap from the cassette tubing. Carefully remove the needle from the syringe (be sure to discard the needle in an appropriate manner) and attach the syringe to the cassette tubing. Hold the cassette in one hand and push the plunger to inject the reconstituted solution into the cassette (alternatively, you may find it useful to use a tabletop or other solid structure to steady the plunger while pushing down on the syringe to inject the solution). When the syringe is empty, clamp the cassette tubing near the syringe. Disconnect the syringe and replace the cassette tubing end cap.
To inject the remaining diluent into the partially filled cassette
Using a 60 mL syringe, attach a new needle to the syringe and follow the above procedures for breaking the syringe seal and wiping the tops of the sterile diluent vials. Fill the syringe with the amount of air that is equal to the amount of sterile diluent you will remove from the first vial. Insert the needle through the rubber seal and slowly inject some of the air into the vial, allowing the fluid to flow into the syringe. Continue to push air gently into the vial until all of the fluid in the vial has flowed into the syringe. Remove any air that may be in the syringe as described above. Allow the pressure to equalize before you pull the needle out or you may lose fluid from the syringe. (If this occurs, the whole process needs to be repeated.) Withdraw the needle and replace the needle cap on the syringe. You may find it easier to hold the larger syringe in an upside down, vertical position while withdrawing the fluid in the vial.
To inject the sterile diluent into the cassette
Uncap the clamped cassette tube and carefully remove the needle from the syringe (discarding the needle in an appropriate manner). Attach the syringe to the cassette tubing. Unclamp the cassette tubing and carefully inject the solution into the cassette. When the syringe is empty, clamp the cassette tube near the syringe and disconnect the syringe. Replace the cap on the cassette tube. If more diluent is needed to fill the cassette, repeat steps 6 and 7 with an additional vial of diluent; however, after completing the transfer of all of the required diluent, clamp the tubing, but leave the syringe attached to the cassette tubing while you mix the solution. Gently turn the cassette upside down at least 10 times to thoroughly mix the reconstituted epoprostenol with the additional diluent.
To remove air from the cassette
To remove the air from inside the cassette, slowly turn the cassette until all of the small bubbles of air join to form one air pocket. Tilt the cassette gently so that the air pocket is in the corner where the tubing connects to the cassette. Unclamp the tube and pull back the plunger of the syringe until you see fluid fill the tubing. Clamp the tube near the connector and remove the syringe and replace the cap on the tubing. Label the cassette with the current time and date. Store the cassette in the refrigerator (preferably, on the top shelf to avoid spilling any food or drink on it) until it is time to use it. Make up a new cassette each day and use the cassette you refrigerated the day before so that you will always have a back-up cassette.
To use the pump
The instructions for the use of the pump may vary depending on the particular make and model of the pump. Your doctor or nurse will give detailed instructions on how to use and care for the particular pump and accessories that you will use for administering your medicine. These instructions should include how to change the pump battery, cassette, and tubing. Remember to change the gel packs every 12 hours or every 8 hours if the surrounding temperature approaches 86 °F.
Maintain sterile technique at all times. If you suspect that you have contaminated anything, throw away the accessories and begin again.
Dosing
The dose of this medicine will be different for different patients. Follow your doctor's orders or the directions on the label. The following information includes only the average doses of this medicine. If your dose is different, do not change it unless your doctor tells you to do so.
The amount of medicine that you take depends on the strength of the medicine. Also, the number of doses you take each day, the time allowed between doses, and the length of time you take the medicine depend on the medical problem for which you are using the medicine.
The amount of medicine that you take depends on the concentration of the reconstituted medicine and the rate at which the infusion pump delivers the medicine.
For injection dosage form:
For primary pulmonary hypertension and pulmonary hypertension secondary to scleroderma spectrum of disease:
Adults—Initially, 2 nanograms per kilogram (kg) (0.9 nanogram per pound) of body weight per minute. Your doctor may increase your dose as necessary.
Children—Use and dose must be determined by your doctor.
Missed Dose
Call your doctor or pharmacist for instructions.
Epoprostenol has to be administered by a continuous intravenous infusion and it must never be stopped suddenly.
Storage
Store the medicine in a closed container at room temperature, away from heat, moisture, and direct light. Keep from freezing.
Keep out of the reach of children.
Do not keep outdated medicine or medicine no longer needed.
Store the reconstituted injection in the refrigerator, away from direct light. However, keep the medicine from freezing. Any medicine that has been frozen should be thrown away. Reconstituted solutions should be kept either in the refrigerator or in a cold pouch, or a combination of the two, for no more than 48 hours. Do not expose reconstituted solution to temperatures higher than 25 °C (77 °F). If the reconstituted solution has particles in it or is discolored, it should be discarded.
Precautions While Using Flolan
It is important that your doctor check your progress at regular visits. This will allow your doctor to make sure the medicine is working properly and to change the dosage if needed.
Be sure to report any signs of infection at the catheter site to your doctor. Also, if you develop a sudden fever, contact your doctor as soon as possible.
Avoid the use of saunas, hot baths, or sunbathing, or other situations that may cause blood vessels to dilate, resulting in low blood pressure and increasing the possibility of dizziness, light-headedness, or fainting.
Do not suddenly stop using this medicine. Stopping suddenly may bring on symptoms of your condition and can be dangerous. Check with your doctor before stopping completely.
Your doctor may want you to carry a medical identification card stating that you are using this medicine.
Flolan Side Effects
Along with its needed effects, a medicine may cause some unwanted effects. Although not all of these side effects may occur, if they do occur they may need medical attention.
Check with your doctor immediately if any of the following side effects occur:
Signs and symptoms that can occur with initial dosage adjustments and/or dosage excess
Diarrhea
fast heartbeat
headache
light-headedness or fainting
nausea
redness of face or neck (flushing)
vomiting
Check with your doctor as soon as possible if any of the following side effects occur:
More common
Anxiety and/or nervousness
diarrhea
dizziness
flu or infection-like symptoms, such as chills, confusion, delirium, light-headedness or fainting, fast heartbeat, fever, and/or rapid, shallow breathing
headache
jaw pain (when chewing)
local infection at the catheter site
pain at injection site
pain in muscles or bones
redness of face (flushing)
unusual bleeding such as nosebleeds or bleeding gums or bruising
Less common
Altered or abnormal touch sensation or sensitivity
After you stop using this medicine, it may still produce some side effects that need attention. During this period of time, check with your doctor immediately if you notice the following side effects:
Difficult or labored breathing
dizziness
fainting
weakness
Other side effects not listed may also occur in some patients. If you notice any other effects, check with your healthcare professional.
用药温馨提示:当您服用此药物时,需定期接受医疗专业人士的检查,以便随时针对其药效、副作用等情况进行监测。本网站所包含的信息旨在为患者提供帮助,不能代替医学建议和治疗。
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 依前列醇钠_FLOLAN_依前列醇钠注射剂说明书
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 依前列醇钠_FLOLAN_依前列醇钠注射剂说明书