艾曲波帕_REVOLADE_艾曲波帕片Eltrombopag说明书
[caption id="attachment_34379" align="alignleft" width="300"]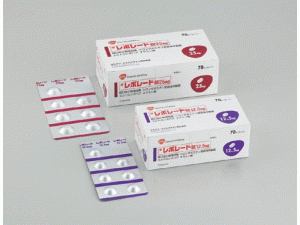 REVOLADE Tablets 25mg(艾曲波帕片 レボレード錠)[/caption]
药店国别:
产地国家:日本
处 方 药:是
所属类别:25毫克/片 70片/盒
包装规格:25毫克/片 70片/盒
计价单位:盒
生产厂家中文参考译名:葛兰素史克
生产厂家英文名:GlaxoSmithKline
原产地英文商品名:Revolade 25MG 70TABLETS.
原产地英文药品名:Eltrombopag OLAMINE
中文参考商品译名:Revolade 25毫克/片 70片/盒
中文参考药品译名:艾曲波帕
简介:
部份中文艾曲波帕处方资料(仅供参考)
英文商品名:Revolade
英文药名:Eltrombopag
中文药名:艾曲波帕
日文药名:レボレード錠
原研公司:葛兰素史克
规 格:
片剂:50mg
剂量和给药方法:
1.对大多数患者的起始剂量是50mg每天1次
2.对东方人患者或中度或严重肝功能不全患者,起始剂量为25mg每天1次。
3.空腹给药(餐前1小时或2小时)。
4.和其它药物、食物或多价阳离子(如,铁、钙、铝、镁、硒,和锌)添加剂间间隔4小时。
5.为减低出血风险调整每天剂量至达到和维持血小板计数≥50×109/L。
6.每天剂量不要超过75mg。
7.如最大剂量后4周血小板计数不增加中断Revolade,重要肝功能检验异常或血小板计数反应过量也中断。
禁忌证:无。
警告和注意事项:
1.肝毒性。观察血清中转氨酶水平和胆红素增加。治疗开始前和治疗期间常规必须测定肝化学。
2.肝功能受损患者给药时小心谨慎。
3.艾曲波帕片是促血小板生成素受体激动剂和TPO-受体激动剂增加骨髓内网状纤维沉积的发展或进展的风险。为骨髓纤维化征象监查外周血。中断可能导致比治疗前存在血小板减少变坏。中断后每周监查全血细胞计数(CBCs),血小板计数至少4周。剂量过量可能增高血小板计数至一个产生血栓形成/血栓栓塞并发症
4.Revolade可能增高血液病恶性病的风险,特别是在骨髓增生异常综合征患者。
5.用Revolade治疗调整剂量期时每周监查CBC,包括血小板计数和外周血图片,然后确定稳定剂量。
最常见不良反应:
恶心、呕吐、月经过多、肌肉痛、感觉异常、白内障、消化不良、瘀斑、血小板减少、ALT/AST增加和结膜出血。
药物相互作用:
1.艾曲波帕是OATP1B1转运蛋白的抑制剂。紧密监查患者过量暴露于OATP1B1底物(如,罗苏伐他汀(rosuvastatin))药物征象和症状并考虑减低这些药物的剂量。
2.多价阳离子(如、铁、钙、铝、镁、硒、和锌)显示减低艾曲波帕的吸收;服药4小时内不建议吃任何含多价阳离子药物如抗酸药、乳制品、和矿物补充剂
特殊人群中的应用:
妊娠:可能引起胎儿伤害。
哺乳母亲:应作出决策中断服药或哺乳。
REVOLADE®
film-coated tablets
Eltrombopag olamine
CONSUMER MEDICINE INFORMATION
NOTICE: This Consumer Medicine Information (CMI) is intended for persons living in Australia. This page contains answers to some common questions about Revolade. It does not contain all the information that is known about Revolade. It does not take the place of talking to your doctor or pharmacist. All medicines have risks and benefits. Your doctor has weighed the risk of you using this medicine against the benefits he/she expects it will have for you. If you have any concerns about using this medicine, ask your doctor or pharmacist. Bookmark or print this page, you may need to read it again.
WHAT REVOLADE IS USED FOR
REVOLADE is a medicine that may help to increase the number of platelets, a type of blood cell that helps to reduce or prevent bleeding.
It may be used to treat a bleeding disorder known as idiopathic thrombocytopenic purpura (ITP). ITP is the condition of having a low platelet count (thrombocytopenia). ITP patients may suffer from an increased risk of bleeding. Symptoms/signs patients with ITP may notice are petechiae (pinpoint sized flat round red spots under the skin), purpura (bruising), nosebleeds, bleeding gums and not being able to control bleeding if cuts or injuries occur.
REVOLADE also may be used to treat patients with hepatitis C virus (HCV) infections for the treatment of thrombocytopenia.
Many patients with HCV infections have low platelet counts (thrombocytopenia) not only as a result of the disease but also due to some of the medicines that are used to treat the disease. The use of REVOLADE to increase and maintain the platelet count prior to and throughout antiviral treatment of HCV infection gives patients a better opportunity to maintain the optimal the dose and duration of their antiviral therapy.
Ask your doctor if you have any questions about why this medicine has been prescribed for you.
Your doctor may have prescribed it for another reason.
BEFORE YOU TAKE REVOLADE
You must not take REVOLADE:
if you are allergic (hypersensitive) to eltrombopag olamine or to any other ingredients of REVOLADE (listed at the end of this leaflet).
Check with your doctor if you think this may apply to you.
Tell your doctor if you:
have liver problems. You may need a lower dose of REVOLADE
have kidney problems
have risk factors for thrombosis (formation of a clot inside a blood vessel, obstructing the flow of blood), or you know that thrombosis occurs frequently in your family
have a history of blood cancers
have had or develop sensitivity to the sun
have a history of cataracts (problems with sight)
are pregnant or plan to become pregnant
are breast feeding.
Taking other medicines
There are certain groups of medicines, including prescription and non-prescription medicines and vitamins that interact with REVOLADE that you should not take at the same time or that require a dose adjustment while receiving a course of REVOLADE. These medications include some products within the following groups:
antacid medicines to treat stomach ulcers or heartburn
certain drugs used to lower cholesterol (statins)
certain drugs used to treat human immunodeficiency virus (HIV) (lopinavir / ritonavir)
minerals such as aluminium, calcium, iron, magnesium, selenium and zinc which may be found in mineral supplements and complementary medicines.
Your doctor will review the medicines you are currently taking to make sure you are not taking something that cannot be taken with the REVOLADE. If you require any of these medications and a suitable substitute is not available, please discuss this with your doctor.
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription.
Taking REVOLADE with food and drink
REVOLADE is affected by calcium intake. REVOLADE may be taken with food low in calcium such as:
fruits such as pineapple, raisins and strawberries
lean ham, chicken or beef
unfortified fruit juice, soy milk and grain. (Unfortified means no added calcium, magnesium or iron).
Please discuss this matter with your doctor; they will be able to advise on the most suitable meals to be eaten while you are taking REVOLADE.
Don't take REVOLADE during the 4 hours before or after you take:
antacid medication
to treat indigestion
mineral supplements,
such as aluminium, calcium, iron, magnesium, selenium or zinc
dairy products.
If you do, the medicine will not be properly absorbed into your body.
One way to avoid issues with these products would be to take them in the morning and REVOLADE in the evening. Ask your doctor or pharmacist for advice if you are unsure.
Pregnancy and breast-feeding
You should avoid becoming pregnant while taking REVOLADE because the effect of REVOLADE on pregnancy is not known. You should use a reliable method of contraception (a way to prevent you from becoming pregnant).
If you become pregnant during treatment, tell your doctor.
Breast feeding is not recommended while you are taking REVOLADE. It is not known whether REVOLADE passes into breast milk.
Ask your doctor or pharmacist for advice before taking any medicine if you are unsure.
Phototoxicity
REVOLADE may cause you to sunburn more easily. As a safety precaution, while taking REVOLADE, you should avoid exposure to high-intensity artificial UV light such as tanning beds and being unprotected when in direct sunlight. If you do need to be in the sun, use protective clothing, sun glasses and sunscreen.
Cataracts
In animal studies it was found that REVOLADE caused the development of cataracts (a clouding of the lens in the eye). In HCV trials in humans an increased risk in the incidence of cataracts has also been seen. Your doctor may recommend that you are checked for cataracts as part of any routine eye examination.
HOW TO TAKE REVOLADE
Always take REVOLADE exactly as your doctor has told you. You should check with your doctor or pharmacist if you are not sure.
How much to take and when to take it
REVOLADE should be taken at least 4 hours before or at least 4 hours after antacids, dairy products or some mineral supplements such as iron, calcium, magnesium, aluminium, selenium and zinc.
One way to avoid issues with these products would be to take them in the morning and REVOLADE in the evening.
The usual starting dose for ITP patients is one 50 mg REVOLADE tablet a day. People of East Asian origin (Chinese, Japanese, Taiwanese, Korean or Thai) need to start at a lower dose of 25 mg.
The usual starting dose for HCV patients is one 25 mg REVOLADE tablet a day. People of East Asian origin (Chinese, Japanese, Taiwanese, Korean or Thai) will start on the same 25 mg dose.
Based on your response to REVOLADE your doctor will adapt the dose and may recommend that your daily dose of REVOLADE be increased or decreased.
Please expect that at the beginning of therapy your platelet count and other routine blood parameters will need to be monitored frequently. Your doctor will also carry out blood tests to check your liver function before and during treatment with REVOLADE.
How to take it
Swallow REVOLADE with a glass of water.
If you take more REVOLADE than you should (Overdose)
Immediately telephone your doctor or the Poisons Information Centre (In Australia call 13 11 26. In New Zealand call 0800 POISON or 0800 764 766) for advice, or go to Accident and Emergency at the nearest hospital, if you think that you or anyone else may have taken too much REVOLADE. Do this even if there are no signs of discomfort or poisoning. If you are not sure what to do, contact your doctor or pharmacist.
If you forget to take REVOLADE
Do not take a double dose to make up for a forgotten dose.
If you have any further questions on the use of REVOLADE, ask your doctor or pharmacist.
Once you have started taking REVOLADE
Do not stop taking REVOLADE before talking to your doctor or pharmacist.
After you have stopped taking REVOLADE, your bleeding symptoms may come back. Tell your doctor or pharmacist if you have any bleeding in the 4 weeks after you stop taking REVOLADE.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
WHAT ARE THE POSSIBLE SIDE-EFFECTS
Like all medicines, REVOLADE can cause side effects, although not everybody gets them.
The following side effects have been reported to be associated with treatment with REVOLADE in patients with ITP.
Very common side effects
These may affect more than 1 in 10 people treated with REVOLADE.
Nausea and diarrhoea
Common side effects
These may affect up to 1 in 10 people treated with REVOLADE.
Increase of liver enzymes called aspartate and alanine transaminases,
Dry mouth
Vomiting
Alopecia (unusual hair loss or thinning)
Rash
Back pain
Musculoskeletal chest pain, musculoskeletal pain (pain that affects muscles and tendons along with bones)
Myalgia (aching muscles)Pharyngitis (sore throat and discomfort when swallowing)
Urinary tract infection (infection in the system that produces urine).
Other side effects
Other side effects have occurred during further studies with REVOLADE
Rare side effects (these may affect up to 1 in 1,000 people):
Clots in small blood vessels, which may harm organs such as the kidneys (Microangiopathy associated with renal impairment)
The following side effects have been reported to be associated with treatment with REVOLADE in combination with peginterferon and ribavirin in patients with HCV.
Very common side effects
These may affect more than 1 in 10 people treated with REVOLADE and antiviral agents.
Fever
Fatigue
Chills
Headache
Cough
Nausea
Diarrhoea
Unusual hair loss or thinning
Muscle pain
Itching
Feeling weak
Difficulty sleeping
Loss of appetite
Flu-like symptoms
Oedema peripheral (swelling of the hands, ankles or feet)
Very common side effects that may show up in blood tests
Anaemia (reduced number of red blood cells)
Common side effects that may show up in blood tests
These may affect up to 1 in 10 people treated with REVOLADE and antiviral agents.
Increase in bilirubin (a substance produced by the liver)
Tell your doctor immediately if you get any of these symptoms. These symptoms may persist after you stop taking REVOLADE.
Liver problems
REVOLADE may damage your liver and cause serious, even life threatening, illness. You must have blood tests to check your liver before you start taking REVOLADE and during treatment. When you are given certain antiviral treatments together with REVOLADE for the treatment of thrombocytopenia due to hepatitis C virus (HCV) infections some liver problems can get worse.
Your doctor will order these blood tests and any other tests required. In some cases REVOLADE treatment may need to be stopped. Tell your doctor right away if you have any of these signs and symptoms of liver problems:
jaundice (yellowing of the skin or the whites of the eyes)
unusual darkening of the urine
unusual tiredness
right upper stomach area pain
Bleeding after you stop treatment
When you stop taking REVOLADE, your blood platelet count will drop back down to what it was before you started taking REVOLADE. These effects are most likely to happen within 4 weeks after you stop taking REVOLADE. The lower platelet counts may increase your risk of bleeding. Your doctor will check your platelet counts for at least 4 weeks after you stop taking REVOLADE. Tell your doctor or pharmacist if you have any bruising or bleeding after you stop taking REVOLADE.
You may have problems with your bone marrow
People with the disease for which you are being treated may have problems with their bone marrow. Medicines like REVOLADE could make this problem worse. Your doctor may also carry out tests to check your bone marrow during treatment with REVOLADE.
High platelet counts and higher chance for blood clots
You have a higher chance of getting a blood clot if your platelet count is too high during treatment with REVOLADE, but blood clots can occur with normal or even low platelet counts. If you have cirrhosis of the liver you are at risk of a blood clot in a blood vessel that feeds your liver (portal vein thrombosis). You may have severe complications from some forms of blood clots, such as clots that travel to the lungs or that cause heart attacks or strokes. Your doctor will check your blood platelet counts, and change your dose or stop REVOLADE if your platelet counts get too high. Tell your doctor right away if you have signs and symptoms of a blood clot in the leg, such as swelling or pain/tenderness of one leg.
If any of the side effects listed in this leaflet get serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
Tell your doctor or pharmacist if you have any bleeding in the 4 weeks after you stop taking REVOLADE.
STORAGE
Do not store above 30°C.
Keep out of the reach and sight of children.
Do not use REVOLADE after the expiry date which is stated on the carton.
Disposal
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required.
PRODUCT DESCRIPTION
What REVOLADE looks like
REVOLADE is presented in packs of 28 tablets.
25 mg tablets
REVOLADE film-coated tablets are round, biconvex, white, debossed with 'GS NX3' and '25' on one side.
50 mg tablets
REVOLADE film-coated tablets are round, biconvex, brown, debossed with 'GS UFU' and '50' on one side.
75 mg tablets
REVOLADE film-coated tablets are round, biconvex, pink, debossed with 'GS FSS' and '75' on one side.
Ingredients
REVOLADE contains the active ingredient eltrombopag olamine.
REVOLADE also contains hypromellose, macrogol 400, magnesium stearate, mannitol, cellulose - microcrystalline, povidone, sodium starch glycollate, titanium dioxide (E171).
25 mg
The 25 mg tablet also contains polysorbate 80.
50 mg
The 50 mg tablet also contains iron oxide red CI77491 (E172) and iron oxide yellow CI77492 (E172).
75 mg
The 75 mg tablet also contains iron oxide black CI77499 and oxide red CI77491 (E172).
SUPPLIER
Your REVOLADE is supplied by:
GlaxoSmithKline Australia Ltd
Level 4, 436 Johnston St,
Abbotsford, Victoria, 3067
AUSTRALIA.
or
GlaxoSmithKline NZ Ltd
Private Bag 106600
Downtown
Auckland 1143
NEW ZEALAND
WHERE TO GO FOR FURTHER INFORMATION
Pharmaceutical companies are not in a position to give people an individual diagnosis or medical advice. Your doctor or pharmacist is the best person to give you the individual advice you need.
In Australia: For more information on Revolade please go to www.gsk.com.au/revolade or your doctor or pharmacist.
In New Zealand: For more information on Revolade please go to your doctor or pharmacist.
This leaflet was prepared on 28 July 2014.
The information provided applies only to REVOLADE®.
REVOLADE is a registered trade mark of the GlaxoSmithKline Group of Companies.
25 mg tablets AUST R 158419
50 mg tablets AUST R 158356
75 mg tablets AUST R 200121
© 2013 GlaxoSmithKline Australia
REVOLADE Tablets 25mg(艾曲波帕片 レボレード錠)[/caption]
药店国别:
产地国家:日本
处 方 药:是
所属类别:25毫克/片 70片/盒
包装规格:25毫克/片 70片/盒
计价单位:盒
生产厂家中文参考译名:葛兰素史克
生产厂家英文名:GlaxoSmithKline
原产地英文商品名:Revolade 25MG 70TABLETS.
原产地英文药品名:Eltrombopag OLAMINE
中文参考商品译名:Revolade 25毫克/片 70片/盒
中文参考药品译名:艾曲波帕
简介:
部份中文艾曲波帕处方资料(仅供参考)
英文商品名:Revolade
英文药名:Eltrombopag
中文药名:艾曲波帕
日文药名:レボレード錠
原研公司:葛兰素史克
规 格:
片剂:50mg
剂量和给药方法:
1.对大多数患者的起始剂量是50mg每天1次
2.对东方人患者或中度或严重肝功能不全患者,起始剂量为25mg每天1次。
3.空腹给药(餐前1小时或2小时)。
4.和其它药物、食物或多价阳离子(如,铁、钙、铝、镁、硒,和锌)添加剂间间隔4小时。
5.为减低出血风险调整每天剂量至达到和维持血小板计数≥50×109/L。
6.每天剂量不要超过75mg。
7.如最大剂量后4周血小板计数不增加中断Revolade,重要肝功能检验异常或血小板计数反应过量也中断。
禁忌证:无。
警告和注意事项:
1.肝毒性。观察血清中转氨酶水平和胆红素增加。治疗开始前和治疗期间常规必须测定肝化学。
2.肝功能受损患者给药时小心谨慎。
3.艾曲波帕片是促血小板生成素受体激动剂和TPO-受体激动剂增加骨髓内网状纤维沉积的发展或进展的风险。为骨髓纤维化征象监查外周血。中断可能导致比治疗前存在血小板减少变坏。中断后每周监查全血细胞计数(CBCs),血小板计数至少4周。剂量过量可能增高血小板计数至一个产生血栓形成/血栓栓塞并发症
4.Revolade可能增高血液病恶性病的风险,特别是在骨髓增生异常综合征患者。
5.用Revolade治疗调整剂量期时每周监查CBC,包括血小板计数和外周血图片,然后确定稳定剂量。
最常见不良反应:
恶心、呕吐、月经过多、肌肉痛、感觉异常、白内障、消化不良、瘀斑、血小板减少、ALT/AST增加和结膜出血。
药物相互作用:
1.艾曲波帕是OATP1B1转运蛋白的抑制剂。紧密监查患者过量暴露于OATP1B1底物(如,罗苏伐他汀(rosuvastatin))药物征象和症状并考虑减低这些药物的剂量。
2.多价阳离子(如、铁、钙、铝、镁、硒、和锌)显示减低艾曲波帕的吸收;服药4小时内不建议吃任何含多价阳离子药物如抗酸药、乳制品、和矿物补充剂
特殊人群中的应用:
妊娠:可能引起胎儿伤害。
哺乳母亲:应作出决策中断服药或哺乳。
REVOLADE®
film-coated tablets
Eltrombopag olamine
CONSUMER MEDICINE INFORMATION
NOTICE: This Consumer Medicine Information (CMI) is intended for persons living in Australia. This page contains answers to some common questions about Revolade. It does not contain all the information that is known about Revolade. It does not take the place of talking to your doctor or pharmacist. All medicines have risks and benefits. Your doctor has weighed the risk of you using this medicine against the benefits he/she expects it will have for you. If you have any concerns about using this medicine, ask your doctor or pharmacist. Bookmark or print this page, you may need to read it again.
WHAT REVOLADE IS USED FOR
REVOLADE is a medicine that may help to increase the number of platelets, a type of blood cell that helps to reduce or prevent bleeding.
It may be used to treat a bleeding disorder known as idiopathic thrombocytopenic purpura (ITP). ITP is the condition of having a low platelet count (thrombocytopenia). ITP patients may suffer from an increased risk of bleeding. Symptoms/signs patients with ITP may notice are petechiae (pinpoint sized flat round red spots under the skin), purpura (bruising), nosebleeds, bleeding gums and not being able to control bleeding if cuts or injuries occur.
REVOLADE also may be used to treat patients with hepatitis C virus (HCV) infections for the treatment of thrombocytopenia.
Many patients with HCV infections have low platelet counts (thrombocytopenia) not only as a result of the disease but also due to some of the medicines that are used to treat the disease. The use of REVOLADE to increase and maintain the platelet count prior to and throughout antiviral treatment of HCV infection gives patients a better opportunity to maintain the optimal the dose and duration of their antiviral therapy.
Ask your doctor if you have any questions about why this medicine has been prescribed for you.
Your doctor may have prescribed it for another reason.
BEFORE YOU TAKE REVOLADE
You must not take REVOLADE:
if you are allergic (hypersensitive) to eltrombopag olamine or to any other ingredients of REVOLADE (listed at the end of this leaflet).
Check with your doctor if you think this may apply to you.
Tell your doctor if you:
have liver problems. You may need a lower dose of REVOLADE
have kidney problems
have risk factors for thrombosis (formation of a clot inside a blood vessel, obstructing the flow of blood), or you know that thrombosis occurs frequently in your family
have a history of blood cancers
have had or develop sensitivity to the sun
have a history of cataracts (problems with sight)
are pregnant or plan to become pregnant
are breast feeding.
Taking other medicines
There are certain groups of medicines, including prescription and non-prescription medicines and vitamins that interact with REVOLADE that you should not take at the same time or that require a dose adjustment while receiving a course of REVOLADE. These medications include some products within the following groups:
antacid medicines to treat stomach ulcers or heartburn
certain drugs used to lower cholesterol (statins)
certain drugs used to treat human immunodeficiency virus (HIV) (lopinavir / ritonavir)
minerals such as aluminium, calcium, iron, magnesium, selenium and zinc which may be found in mineral supplements and complementary medicines.
Your doctor will review the medicines you are currently taking to make sure you are not taking something that cannot be taken with the REVOLADE. If you require any of these medications and a suitable substitute is not available, please discuss this with your doctor.
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription.
Taking REVOLADE with food and drink
REVOLADE is affected by calcium intake. REVOLADE may be taken with food low in calcium such as:
fruits such as pineapple, raisins and strawberries
lean ham, chicken or beef
unfortified fruit juice, soy milk and grain. (Unfortified means no added calcium, magnesium or iron).
Please discuss this matter with your doctor; they will be able to advise on the most suitable meals to be eaten while you are taking REVOLADE.
Don't take REVOLADE during the 4 hours before or after you take:
antacid medication
to treat indigestion
mineral supplements,
such as aluminium, calcium, iron, magnesium, selenium or zinc
dairy products.
If you do, the medicine will not be properly absorbed into your body.
One way to avoid issues with these products would be to take them in the morning and REVOLADE in the evening. Ask your doctor or pharmacist for advice if you are unsure.
Pregnancy and breast-feeding
You should avoid becoming pregnant while taking REVOLADE because the effect of REVOLADE on pregnancy is not known. You should use a reliable method of contraception (a way to prevent you from becoming pregnant).
If you become pregnant during treatment, tell your doctor.
Breast feeding is not recommended while you are taking REVOLADE. It is not known whether REVOLADE passes into breast milk.
Ask your doctor or pharmacist for advice before taking any medicine if you are unsure.
Phototoxicity
REVOLADE may cause you to sunburn more easily. As a safety precaution, while taking REVOLADE, you should avoid exposure to high-intensity artificial UV light such as tanning beds and being unprotected when in direct sunlight. If you do need to be in the sun, use protective clothing, sun glasses and sunscreen.
Cataracts
In animal studies it was found that REVOLADE caused the development of cataracts (a clouding of the lens in the eye). In HCV trials in humans an increased risk in the incidence of cataracts has also been seen. Your doctor may recommend that you are checked for cataracts as part of any routine eye examination.
HOW TO TAKE REVOLADE
Always take REVOLADE exactly as your doctor has told you. You should check with your doctor or pharmacist if you are not sure.
How much to take and when to take it
REVOLADE should be taken at least 4 hours before or at least 4 hours after antacids, dairy products or some mineral supplements such as iron, calcium, magnesium, aluminium, selenium and zinc.
One way to avoid issues with these products would be to take them in the morning and REVOLADE in the evening.
The usual starting dose for ITP patients is one 50 mg REVOLADE tablet a day. People of East Asian origin (Chinese, Japanese, Taiwanese, Korean or Thai) need to start at a lower dose of 25 mg.
The usual starting dose for HCV patients is one 25 mg REVOLADE tablet a day. People of East Asian origin (Chinese, Japanese, Taiwanese, Korean or Thai) will start on the same 25 mg dose.
Based on your response to REVOLADE your doctor will adapt the dose and may recommend that your daily dose of REVOLADE be increased or decreased.
Please expect that at the beginning of therapy your platelet count and other routine blood parameters will need to be monitored frequently. Your doctor will also carry out blood tests to check your liver function before and during treatment with REVOLADE.
How to take it
Swallow REVOLADE with a glass of water.
If you take more REVOLADE than you should (Overdose)
Immediately telephone your doctor or the Poisons Information Centre (In Australia call 13 11 26. In New Zealand call 0800 POISON or 0800 764 766) for advice, or go to Accident and Emergency at the nearest hospital, if you think that you or anyone else may have taken too much REVOLADE. Do this even if there are no signs of discomfort or poisoning. If you are not sure what to do, contact your doctor or pharmacist.
If you forget to take REVOLADE
Do not take a double dose to make up for a forgotten dose.
If you have any further questions on the use of REVOLADE, ask your doctor or pharmacist.
Once you have started taking REVOLADE
Do not stop taking REVOLADE before talking to your doctor or pharmacist.
After you have stopped taking REVOLADE, your bleeding symptoms may come back. Tell your doctor or pharmacist if you have any bleeding in the 4 weeks after you stop taking REVOLADE.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
WHAT ARE THE POSSIBLE SIDE-EFFECTS
Like all medicines, REVOLADE can cause side effects, although not everybody gets them.
The following side effects have been reported to be associated with treatment with REVOLADE in patients with ITP.
Very common side effects
These may affect more than 1 in 10 people treated with REVOLADE.
Nausea and diarrhoea
Common side effects
These may affect up to 1 in 10 people treated with REVOLADE.
Increase of liver enzymes called aspartate and alanine transaminases,
Dry mouth
Vomiting
Alopecia (unusual hair loss or thinning)
Rash
Back pain
Musculoskeletal chest pain, musculoskeletal pain (pain that affects muscles and tendons along with bones)
Myalgia (aching muscles)Pharyngitis (sore throat and discomfort when swallowing)
Urinary tract infection (infection in the system that produces urine).
Other side effects
Other side effects have occurred during further studies with REVOLADE
Rare side effects (these may affect up to 1 in 1,000 people):
Clots in small blood vessels, which may harm organs such as the kidneys (Microangiopathy associated with renal impairment)
The following side effects have been reported to be associated with treatment with REVOLADE in combination with peginterferon and ribavirin in patients with HCV.
Very common side effects
These may affect more than 1 in 10 people treated with REVOLADE and antiviral agents.
Fever
Fatigue
Chills
Headache
Cough
Nausea
Diarrhoea
Unusual hair loss or thinning
Muscle pain
Itching
Feeling weak
Difficulty sleeping
Loss of appetite
Flu-like symptoms
Oedema peripheral (swelling of the hands, ankles or feet)
Very common side effects that may show up in blood tests
Anaemia (reduced number of red blood cells)
Common side effects that may show up in blood tests
These may affect up to 1 in 10 people treated with REVOLADE and antiviral agents.
Increase in bilirubin (a substance produced by the liver)
Tell your doctor immediately if you get any of these symptoms. These symptoms may persist after you stop taking REVOLADE.
Liver problems
REVOLADE may damage your liver and cause serious, even life threatening, illness. You must have blood tests to check your liver before you start taking REVOLADE and during treatment. When you are given certain antiviral treatments together with REVOLADE for the treatment of thrombocytopenia due to hepatitis C virus (HCV) infections some liver problems can get worse.
Your doctor will order these blood tests and any other tests required. In some cases REVOLADE treatment may need to be stopped. Tell your doctor right away if you have any of these signs and symptoms of liver problems:
jaundice (yellowing of the skin or the whites of the eyes)
unusual darkening of the urine
unusual tiredness
right upper stomach area pain
Bleeding after you stop treatment
When you stop taking REVOLADE, your blood platelet count will drop back down to what it was before you started taking REVOLADE. These effects are most likely to happen within 4 weeks after you stop taking REVOLADE. The lower platelet counts may increase your risk of bleeding. Your doctor will check your platelet counts for at least 4 weeks after you stop taking REVOLADE. Tell your doctor or pharmacist if you have any bruising or bleeding after you stop taking REVOLADE.
You may have problems with your bone marrow
People with the disease for which you are being treated may have problems with their bone marrow. Medicines like REVOLADE could make this problem worse. Your doctor may also carry out tests to check your bone marrow during treatment with REVOLADE.
High platelet counts and higher chance for blood clots
You have a higher chance of getting a blood clot if your platelet count is too high during treatment with REVOLADE, but blood clots can occur with normal or even low platelet counts. If you have cirrhosis of the liver you are at risk of a blood clot in a blood vessel that feeds your liver (portal vein thrombosis). You may have severe complications from some forms of blood clots, such as clots that travel to the lungs or that cause heart attacks or strokes. Your doctor will check your blood platelet counts, and change your dose or stop REVOLADE if your platelet counts get too high. Tell your doctor right away if you have signs and symptoms of a blood clot in the leg, such as swelling or pain/tenderness of one leg.
If any of the side effects listed in this leaflet get serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
Tell your doctor or pharmacist if you have any bleeding in the 4 weeks after you stop taking REVOLADE.
STORAGE
Do not store above 30°C.
Keep out of the reach and sight of children.
Do not use REVOLADE after the expiry date which is stated on the carton.
Disposal
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required.
PRODUCT DESCRIPTION
What REVOLADE looks like
REVOLADE is presented in packs of 28 tablets.
25 mg tablets
REVOLADE film-coated tablets are round, biconvex, white, debossed with 'GS NX3' and '25' on one side.
50 mg tablets
REVOLADE film-coated tablets are round, biconvex, brown, debossed with 'GS UFU' and '50' on one side.
75 mg tablets
REVOLADE film-coated tablets are round, biconvex, pink, debossed with 'GS FSS' and '75' on one side.
Ingredients
REVOLADE contains the active ingredient eltrombopag olamine.
REVOLADE also contains hypromellose, macrogol 400, magnesium stearate, mannitol, cellulose - microcrystalline, povidone, sodium starch glycollate, titanium dioxide (E171).
25 mg
The 25 mg tablet also contains polysorbate 80.
50 mg
The 50 mg tablet also contains iron oxide red CI77491 (E172) and iron oxide yellow CI77492 (E172).
75 mg
The 75 mg tablet also contains iron oxide black CI77499 and oxide red CI77491 (E172).
SUPPLIER
Your REVOLADE is supplied by:
GlaxoSmithKline Australia Ltd
Level 4, 436 Johnston St,
Abbotsford, Victoria, 3067
AUSTRALIA.
or
GlaxoSmithKline NZ Ltd
Private Bag 106600
Downtown
Auckland 1143
NEW ZEALAND
WHERE TO GO FOR FURTHER INFORMATION
Pharmaceutical companies are not in a position to give people an individual diagnosis or medical advice. Your doctor or pharmacist is the best person to give you the individual advice you need.
In Australia: For more information on Revolade please go to www.gsk.com.au/revolade or your doctor or pharmacist.
In New Zealand: For more information on Revolade please go to your doctor or pharmacist.
This leaflet was prepared on 28 July 2014.
The information provided applies only to REVOLADE®.
REVOLADE is a registered trade mark of the GlaxoSmithKline Group of Companies.
25 mg tablets AUST R 158419
50 mg tablets AUST R 158356
75 mg tablets AUST R 200121
© 2013 GlaxoSmithKline Australia
 REVOLADE Tablets 25mg(艾曲波帕片 レボレード錠)[/caption]
药店国别:
产地国家:日本
处 方 药:是
所属类别:25毫克/片 70片/盒
包装规格:25毫克/片 70片/盒
计价单位:盒
生产厂家中文参考译名:葛兰素史克
生产厂家英文名:GlaxoSmithKline
原产地英文商品名:Revolade 25MG 70TABLETS.
原产地英文药品名:Eltrombopag OLAMINE
中文参考商品译名:Revolade 25毫克/片 70片/盒
中文参考药品译名:艾曲波帕
简介:
部份中文艾曲波帕处方资料(仅供参考)
英文商品名:Revolade
英文药名:Eltrombopag
中文药名:艾曲波帕
日文药名:レボレード錠
原研公司:葛兰素史克
规 格:
片剂:50mg
剂量和给药方法:
1.对大多数患者的起始剂量是50mg每天1次
2.对东方人患者或中度或严重肝功能不全患者,起始剂量为25mg每天1次。
3.空腹给药(餐前1小时或2小时)。
4.和其它药物、食物或多价阳离子(如,铁、钙、铝、镁、硒,和锌)添加剂间间隔4小时。
5.为减低出血风险调整每天剂量至达到和维持血小板计数≥50×109/L。
6.每天剂量不要超过75mg。
7.如最大剂量后4周血小板计数不增加中断Revolade,重要肝功能检验异常或血小板计数反应过量也中断。
禁忌证:无。
警告和注意事项:
1.肝毒性。观察血清中转氨酶水平和胆红素增加。治疗开始前和治疗期间常规必须测定肝化学。
2.肝功能受损患者给药时小心谨慎。
3.艾曲波帕片是促血小板生成素受体激动剂和TPO-受体激动剂增加骨髓内网状纤维沉积的发展或进展的风险。为骨髓纤维化征象监查外周血。中断可能导致比治疗前存在血小板减少变坏。中断后每周监查全血细胞计数(CBCs),血小板计数至少4周。剂量过量可能增高血小板计数至一个产生血栓形成/血栓栓塞并发症
4.Revolade可能增高血液病恶性病的风险,特别是在骨髓增生异常综合征患者。
5.用Revolade治疗调整剂量期时每周监查CBC,包括血小板计数和外周血图片,然后确定稳定剂量。
最常见不良反应:
恶心、呕吐、月经过多、肌肉痛、感觉异常、白内障、消化不良、瘀斑、血小板减少、ALT/AST增加和结膜出血。
药物相互作用:
1.艾曲波帕是OATP1B1转运蛋白的抑制剂。紧密监查患者过量暴露于OATP1B1底物(如,罗苏伐他汀(rosuvastatin))药物征象和症状并考虑减低这些药物的剂量。
2.多价阳离子(如、铁、钙、铝、镁、硒、和锌)显示减低艾曲波帕的吸收;服药4小时内不建议吃任何含多价阳离子药物如抗酸药、乳制品、和矿物补充剂
特殊人群中的应用:
妊娠:可能引起胎儿伤害。
哺乳母亲:应作出决策中断服药或哺乳。
REVOLADE®
film-coated tablets
Eltrombopag olamine
CONSUMER MEDICINE INFORMATION
NOTICE: This Consumer Medicine Information (CMI) is intended for persons living in Australia. This page contains answers to some common questions about Revolade. It does not contain all the information that is known about Revolade. It does not take the place of talking to your doctor or pharmacist. All medicines have risks and benefits. Your doctor has weighed the risk of you using this medicine against the benefits he/she expects it will have for you. If you have any concerns about using this medicine, ask your doctor or pharmacist. Bookmark or print this page, you may need to read it again.
WHAT REVOLADE IS USED FOR
REVOLADE is a medicine that may help to increase the number of platelets, a type of blood cell that helps to reduce or prevent bleeding.
It may be used to treat a bleeding disorder known as idiopathic thrombocytopenic purpura (ITP). ITP is the condition of having a low platelet count (thrombocytopenia). ITP patients may suffer from an increased risk of bleeding. Symptoms/signs patients with ITP may notice are petechiae (pinpoint sized flat round red spots under the skin), purpura (bruising), nosebleeds, bleeding gums and not being able to control bleeding if cuts or injuries occur.
REVOLADE also may be used to treat patients with hepatitis C virus (HCV) infections for the treatment of thrombocytopenia.
Many patients with HCV infections have low platelet counts (thrombocytopenia) not only as a result of the disease but also due to some of the medicines that are used to treat the disease. The use of REVOLADE to increase and maintain the platelet count prior to and throughout antiviral treatment of HCV infection gives patients a better opportunity to maintain the optimal the dose and duration of their antiviral therapy.
Ask your doctor if you have any questions about why this medicine has been prescribed for you.
Your doctor may have prescribed it for another reason.
BEFORE YOU TAKE REVOLADE
You must not take REVOLADE:
if you are allergic (hypersensitive) to eltrombopag olamine or to any other ingredients of REVOLADE (listed at the end of this leaflet).
Check with your doctor if you think this may apply to you.
Tell your doctor if you:
have liver problems. You may need a lower dose of REVOLADE
have kidney problems
have risk factors for thrombosis (formation of a clot inside a blood vessel, obstructing the flow of blood), or you know that thrombosis occurs frequently in your family
have a history of blood cancers
have had or develop sensitivity to the sun
have a history of cataracts (problems with sight)
are pregnant or plan to become pregnant
are breast feeding.
Taking other medicines
There are certain groups of medicines, including prescription and non-prescription medicines and vitamins that interact with REVOLADE that you should not take at the same time or that require a dose adjustment while receiving a course of REVOLADE. These medications include some products within the following groups:
antacid medicines to treat stomach ulcers or heartburn
certain drugs used to lower cholesterol (statins)
certain drugs used to treat human immunodeficiency virus (HIV) (lopinavir / ritonavir)
minerals such as aluminium, calcium, iron, magnesium, selenium and zinc which may be found in mineral supplements and complementary medicines.
Your doctor will review the medicines you are currently taking to make sure you are not taking something that cannot be taken with the REVOLADE. If you require any of these medications and a suitable substitute is not available, please discuss this with your doctor.
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription.
Taking REVOLADE with food and drink
REVOLADE is affected by calcium intake. REVOLADE may be taken with food low in calcium such as:
fruits such as pineapple, raisins and strawberries
lean ham, chicken or beef
unfortified fruit juice, soy milk and grain. (Unfortified means no added calcium, magnesium or iron).
Please discuss this matter with your doctor; they will be able to advise on the most suitable meals to be eaten while you are taking REVOLADE.
Don't take REVOLADE during the 4 hours before or after you take:
antacid medication
to treat indigestion
mineral supplements,
such as aluminium, calcium, iron, magnesium, selenium or zinc
dairy products.
If you do, the medicine will not be properly absorbed into your body.
One way to avoid issues with these products would be to take them in the morning and REVOLADE in the evening. Ask your doctor or pharmacist for advice if you are unsure.
Pregnancy and breast-feeding
You should avoid becoming pregnant while taking REVOLADE because the effect of REVOLADE on pregnancy is not known. You should use a reliable method of contraception (a way to prevent you from becoming pregnant).
If you become pregnant during treatment, tell your doctor.
Breast feeding is not recommended while you are taking REVOLADE. It is not known whether REVOLADE passes into breast milk.
Ask your doctor or pharmacist for advice before taking any medicine if you are unsure.
Phototoxicity
REVOLADE may cause you to sunburn more easily. As a safety precaution, while taking REVOLADE, you should avoid exposure to high-intensity artificial UV light such as tanning beds and being unprotected when in direct sunlight. If you do need to be in the sun, use protective clothing, sun glasses and sunscreen.
Cataracts
In animal studies it was found that REVOLADE caused the development of cataracts (a clouding of the lens in the eye). In HCV trials in humans an increased risk in the incidence of cataracts has also been seen. Your doctor may recommend that you are checked for cataracts as part of any routine eye examination.
HOW TO TAKE REVOLADE
Always take REVOLADE exactly as your doctor has told you. You should check with your doctor or pharmacist if you are not sure.
How much to take and when to take it
REVOLADE should be taken at least 4 hours before or at least 4 hours after antacids, dairy products or some mineral supplements such as iron, calcium, magnesium, aluminium, selenium and zinc.
One way to avoid issues with these products would be to take them in the morning and REVOLADE in the evening.
The usual starting dose for ITP patients is one 50 mg REVOLADE tablet a day. People of East Asian origin (Chinese, Japanese, Taiwanese, Korean or Thai) need to start at a lower dose of 25 mg.
The usual starting dose for HCV patients is one 25 mg REVOLADE tablet a day. People of East Asian origin (Chinese, Japanese, Taiwanese, Korean or Thai) will start on the same 25 mg dose.
Based on your response to REVOLADE your doctor will adapt the dose and may recommend that your daily dose of REVOLADE be increased or decreased.
Please expect that at the beginning of therapy your platelet count and other routine blood parameters will need to be monitored frequently. Your doctor will also carry out blood tests to check your liver function before and during treatment with REVOLADE.
How to take it
Swallow REVOLADE with a glass of water.
If you take more REVOLADE than you should (Overdose)
Immediately telephone your doctor or the Poisons Information Centre (In Australia call 13 11 26. In New Zealand call 0800 POISON or 0800 764 766) for advice, or go to Accident and Emergency at the nearest hospital, if you think that you or anyone else may have taken too much REVOLADE. Do this even if there are no signs of discomfort or poisoning. If you are not sure what to do, contact your doctor or pharmacist.
If you forget to take REVOLADE
Do not take a double dose to make up for a forgotten dose.
If you have any further questions on the use of REVOLADE, ask your doctor or pharmacist.
Once you have started taking REVOLADE
Do not stop taking REVOLADE before talking to your doctor or pharmacist.
After you have stopped taking REVOLADE, your bleeding symptoms may come back. Tell your doctor or pharmacist if you have any bleeding in the 4 weeks after you stop taking REVOLADE.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
WHAT ARE THE POSSIBLE SIDE-EFFECTS
Like all medicines, REVOLADE can cause side effects, although not everybody gets them.
The following side effects have been reported to be associated with treatment with REVOLADE in patients with ITP.
Very common side effects
These may affect more than 1 in 10 people treated with REVOLADE.
Nausea and diarrhoea
Common side effects
These may affect up to 1 in 10 people treated with REVOLADE.
Increase of liver enzymes called aspartate and alanine transaminases,
Dry mouth
Vomiting
Alopecia (unusual hair loss or thinning)
Rash
Back pain
Musculoskeletal chest pain, musculoskeletal pain (pain that affects muscles and tendons along with bones)
Myalgia (aching muscles)Pharyngitis (sore throat and discomfort when swallowing)
Urinary tract infection (infection in the system that produces urine).
Other side effects
Other side effects have occurred during further studies with REVOLADE
Rare side effects (these may affect up to 1 in 1,000 people):
Clots in small blood vessels, which may harm organs such as the kidneys (Microangiopathy associated with renal impairment)
The following side effects have been reported to be associated with treatment with REVOLADE in combination with peginterferon and ribavirin in patients with HCV.
Very common side effects
These may affect more than 1 in 10 people treated with REVOLADE and antiviral agents.
Fever
Fatigue
Chills
Headache
Cough
Nausea
Diarrhoea
Unusual hair loss or thinning
Muscle pain
Itching
Feeling weak
Difficulty sleeping
Loss of appetite
Flu-like symptoms
Oedema peripheral (swelling of the hands, ankles or feet)
Very common side effects that may show up in blood tests
Anaemia (reduced number of red blood cells)
Common side effects that may show up in blood tests
These may affect up to 1 in 10 people treated with REVOLADE and antiviral agents.
Increase in bilirubin (a substance produced by the liver)
Tell your doctor immediately if you get any of these symptoms. These symptoms may persist after you stop taking REVOLADE.
Liver problems
REVOLADE may damage your liver and cause serious, even life threatening, illness. You must have blood tests to check your liver before you start taking REVOLADE and during treatment. When you are given certain antiviral treatments together with REVOLADE for the treatment of thrombocytopenia due to hepatitis C virus (HCV) infections some liver problems can get worse.
Your doctor will order these blood tests and any other tests required. In some cases REVOLADE treatment may need to be stopped. Tell your doctor right away if you have any of these signs and symptoms of liver problems:
jaundice (yellowing of the skin or the whites of the eyes)
unusual darkening of the urine
unusual tiredness
right upper stomach area pain
Bleeding after you stop treatment
When you stop taking REVOLADE, your blood platelet count will drop back down to what it was before you started taking REVOLADE. These effects are most likely to happen within 4 weeks after you stop taking REVOLADE. The lower platelet counts may increase your risk of bleeding. Your doctor will check your platelet counts for at least 4 weeks after you stop taking REVOLADE. Tell your doctor or pharmacist if you have any bruising or bleeding after you stop taking REVOLADE.
You may have problems with your bone marrow
People with the disease for which you are being treated may have problems with their bone marrow. Medicines like REVOLADE could make this problem worse. Your doctor may also carry out tests to check your bone marrow during treatment with REVOLADE.
High platelet counts and higher chance for blood clots
You have a higher chance of getting a blood clot if your platelet count is too high during treatment with REVOLADE, but blood clots can occur with normal or even low platelet counts. If you have cirrhosis of the liver you are at risk of a blood clot in a blood vessel that feeds your liver (portal vein thrombosis). You may have severe complications from some forms of blood clots, such as clots that travel to the lungs or that cause heart attacks or strokes. Your doctor will check your blood platelet counts, and change your dose or stop REVOLADE if your platelet counts get too high. Tell your doctor right away if you have signs and symptoms of a blood clot in the leg, such as swelling or pain/tenderness of one leg.
If any of the side effects listed in this leaflet get serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
Tell your doctor or pharmacist if you have any bleeding in the 4 weeks after you stop taking REVOLADE.
STORAGE
Do not store above 30°C.
Keep out of the reach and sight of children.
Do not use REVOLADE after the expiry date which is stated on the carton.
Disposal
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required.
PRODUCT DESCRIPTION
What REVOLADE looks like
REVOLADE is presented in packs of 28 tablets.
25 mg tablets
REVOLADE film-coated tablets are round, biconvex, white, debossed with 'GS NX3' and '25' on one side.
50 mg tablets
REVOLADE film-coated tablets are round, biconvex, brown, debossed with 'GS UFU' and '50' on one side.
75 mg tablets
REVOLADE film-coated tablets are round, biconvex, pink, debossed with 'GS FSS' and '75' on one side.
Ingredients
REVOLADE contains the active ingredient eltrombopag olamine.
REVOLADE also contains hypromellose, macrogol 400, magnesium stearate, mannitol, cellulose - microcrystalline, povidone, sodium starch glycollate, titanium dioxide (E171).
25 mg
The 25 mg tablet also contains polysorbate 80.
50 mg
The 50 mg tablet also contains iron oxide red CI77491 (E172) and iron oxide yellow CI77492 (E172).
75 mg
The 75 mg tablet also contains iron oxide black CI77499 and oxide red CI77491 (E172).
SUPPLIER
Your REVOLADE is supplied by:
GlaxoSmithKline Australia Ltd
Level 4, 436 Johnston St,
Abbotsford, Victoria, 3067
AUSTRALIA.
or
GlaxoSmithKline NZ Ltd
Private Bag 106600
Downtown
Auckland 1143
NEW ZEALAND
WHERE TO GO FOR FURTHER INFORMATION
Pharmaceutical companies are not in a position to give people an individual diagnosis or medical advice. Your doctor or pharmacist is the best person to give you the individual advice you need.
In Australia: For more information on Revolade please go to www.gsk.com.au/revolade or your doctor or pharmacist.
In New Zealand: For more information on Revolade please go to your doctor or pharmacist.
This leaflet was prepared on 28 July 2014.
The information provided applies only to REVOLADE®.
REVOLADE is a registered trade mark of the GlaxoSmithKline Group of Companies.
25 mg tablets AUST R 158419
50 mg tablets AUST R 158356
75 mg tablets AUST R 200121
© 2013 GlaxoSmithKline Australia
REVOLADE Tablets 25mg(艾曲波帕片 レボレード錠)[/caption]
药店国别:
产地国家:日本
处 方 药:是
所属类别:25毫克/片 70片/盒
包装规格:25毫克/片 70片/盒
计价单位:盒
生产厂家中文参考译名:葛兰素史克
生产厂家英文名:GlaxoSmithKline
原产地英文商品名:Revolade 25MG 70TABLETS.
原产地英文药品名:Eltrombopag OLAMINE
中文参考商品译名:Revolade 25毫克/片 70片/盒
中文参考药品译名:艾曲波帕
简介:
部份中文艾曲波帕处方资料(仅供参考)
英文商品名:Revolade
英文药名:Eltrombopag
中文药名:艾曲波帕
日文药名:レボレード錠
原研公司:葛兰素史克
规 格:
片剂:50mg
剂量和给药方法:
1.对大多数患者的起始剂量是50mg每天1次
2.对东方人患者或中度或严重肝功能不全患者,起始剂量为25mg每天1次。
3.空腹给药(餐前1小时或2小时)。
4.和其它药物、食物或多价阳离子(如,铁、钙、铝、镁、硒,和锌)添加剂间间隔4小时。
5.为减低出血风险调整每天剂量至达到和维持血小板计数≥50×109/L。
6.每天剂量不要超过75mg。
7.如最大剂量后4周血小板计数不增加中断Revolade,重要肝功能检验异常或血小板计数反应过量也中断。
禁忌证:无。
警告和注意事项:
1.肝毒性。观察血清中转氨酶水平和胆红素增加。治疗开始前和治疗期间常规必须测定肝化学。
2.肝功能受损患者给药时小心谨慎。
3.艾曲波帕片是促血小板生成素受体激动剂和TPO-受体激动剂增加骨髓内网状纤维沉积的发展或进展的风险。为骨髓纤维化征象监查外周血。中断可能导致比治疗前存在血小板减少变坏。中断后每周监查全血细胞计数(CBCs),血小板计数至少4周。剂量过量可能增高血小板计数至一个产生血栓形成/血栓栓塞并发症
4.Revolade可能增高血液病恶性病的风险,特别是在骨髓增生异常综合征患者。
5.用Revolade治疗调整剂量期时每周监查CBC,包括血小板计数和外周血图片,然后确定稳定剂量。
最常见不良反应:
恶心、呕吐、月经过多、肌肉痛、感觉异常、白内障、消化不良、瘀斑、血小板减少、ALT/AST增加和结膜出血。
药物相互作用:
1.艾曲波帕是OATP1B1转运蛋白的抑制剂。紧密监查患者过量暴露于OATP1B1底物(如,罗苏伐他汀(rosuvastatin))药物征象和症状并考虑减低这些药物的剂量。
2.多价阳离子(如、铁、钙、铝、镁、硒、和锌)显示减低艾曲波帕的吸收;服药4小时内不建议吃任何含多价阳离子药物如抗酸药、乳制品、和矿物补充剂
特殊人群中的应用:
妊娠:可能引起胎儿伤害。
哺乳母亲:应作出决策中断服药或哺乳。
REVOLADE®
film-coated tablets
Eltrombopag olamine
CONSUMER MEDICINE INFORMATION
NOTICE: This Consumer Medicine Information (CMI) is intended for persons living in Australia. This page contains answers to some common questions about Revolade. It does not contain all the information that is known about Revolade. It does not take the place of talking to your doctor or pharmacist. All medicines have risks and benefits. Your doctor has weighed the risk of you using this medicine against the benefits he/she expects it will have for you. If you have any concerns about using this medicine, ask your doctor or pharmacist. Bookmark or print this page, you may need to read it again.
WHAT REVOLADE IS USED FOR
REVOLADE is a medicine that may help to increase the number of platelets, a type of blood cell that helps to reduce or prevent bleeding.
It may be used to treat a bleeding disorder known as idiopathic thrombocytopenic purpura (ITP). ITP is the condition of having a low platelet count (thrombocytopenia). ITP patients may suffer from an increased risk of bleeding. Symptoms/signs patients with ITP may notice are petechiae (pinpoint sized flat round red spots under the skin), purpura (bruising), nosebleeds, bleeding gums and not being able to control bleeding if cuts or injuries occur.
REVOLADE also may be used to treat patients with hepatitis C virus (HCV) infections for the treatment of thrombocytopenia.
Many patients with HCV infections have low platelet counts (thrombocytopenia) not only as a result of the disease but also due to some of the medicines that are used to treat the disease. The use of REVOLADE to increase and maintain the platelet count prior to and throughout antiviral treatment of HCV infection gives patients a better opportunity to maintain the optimal the dose and duration of their antiviral therapy.
Ask your doctor if you have any questions about why this medicine has been prescribed for you.
Your doctor may have prescribed it for another reason.
BEFORE YOU TAKE REVOLADE
You must not take REVOLADE:
if you are allergic (hypersensitive) to eltrombopag olamine or to any other ingredients of REVOLADE (listed at the end of this leaflet).
Check with your doctor if you think this may apply to you.
Tell your doctor if you:
have liver problems. You may need a lower dose of REVOLADE
have kidney problems
have risk factors for thrombosis (formation of a clot inside a blood vessel, obstructing the flow of blood), or you know that thrombosis occurs frequently in your family
have a history of blood cancers
have had or develop sensitivity to the sun
have a history of cataracts (problems with sight)
are pregnant or plan to become pregnant
are breast feeding.
Taking other medicines
There are certain groups of medicines, including prescription and non-prescription medicines and vitamins that interact with REVOLADE that you should not take at the same time or that require a dose adjustment while receiving a course of REVOLADE. These medications include some products within the following groups:
antacid medicines to treat stomach ulcers or heartburn
certain drugs used to lower cholesterol (statins)
certain drugs used to treat human immunodeficiency virus (HIV) (lopinavir / ritonavir)
minerals such as aluminium, calcium, iron, magnesium, selenium and zinc which may be found in mineral supplements and complementary medicines.
Your doctor will review the medicines you are currently taking to make sure you are not taking something that cannot be taken with the REVOLADE. If you require any of these medications and a suitable substitute is not available, please discuss this with your doctor.
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription.
Taking REVOLADE with food and drink
REVOLADE is affected by calcium intake. REVOLADE may be taken with food low in calcium such as:
fruits such as pineapple, raisins and strawberries
lean ham, chicken or beef
unfortified fruit juice, soy milk and grain. (Unfortified means no added calcium, magnesium or iron).
Please discuss this matter with your doctor; they will be able to advise on the most suitable meals to be eaten while you are taking REVOLADE.
Don't take REVOLADE during the 4 hours before or after you take:
antacid medication
to treat indigestion
mineral supplements,
such as aluminium, calcium, iron, magnesium, selenium or zinc
dairy products.
If you do, the medicine will not be properly absorbed into your body.
One way to avoid issues with these products would be to take them in the morning and REVOLADE in the evening. Ask your doctor or pharmacist for advice if you are unsure.
Pregnancy and breast-feeding
You should avoid becoming pregnant while taking REVOLADE because the effect of REVOLADE on pregnancy is not known. You should use a reliable method of contraception (a way to prevent you from becoming pregnant).
If you become pregnant during treatment, tell your doctor.
Breast feeding is not recommended while you are taking REVOLADE. It is not known whether REVOLADE passes into breast milk.
Ask your doctor or pharmacist for advice before taking any medicine if you are unsure.
Phototoxicity
REVOLADE may cause you to sunburn more easily. As a safety precaution, while taking REVOLADE, you should avoid exposure to high-intensity artificial UV light such as tanning beds and being unprotected when in direct sunlight. If you do need to be in the sun, use protective clothing, sun glasses and sunscreen.
Cataracts
In animal studies it was found that REVOLADE caused the development of cataracts (a clouding of the lens in the eye). In HCV trials in humans an increased risk in the incidence of cataracts has also been seen. Your doctor may recommend that you are checked for cataracts as part of any routine eye examination.
HOW TO TAKE REVOLADE
Always take REVOLADE exactly as your doctor has told you. You should check with your doctor or pharmacist if you are not sure.
How much to take and when to take it
REVOLADE should be taken at least 4 hours before or at least 4 hours after antacids, dairy products or some mineral supplements such as iron, calcium, magnesium, aluminium, selenium and zinc.
One way to avoid issues with these products would be to take them in the morning and REVOLADE in the evening.
The usual starting dose for ITP patients is one 50 mg REVOLADE tablet a day. People of East Asian origin (Chinese, Japanese, Taiwanese, Korean or Thai) need to start at a lower dose of 25 mg.
The usual starting dose for HCV patients is one 25 mg REVOLADE tablet a day. People of East Asian origin (Chinese, Japanese, Taiwanese, Korean or Thai) will start on the same 25 mg dose.
Based on your response to REVOLADE your doctor will adapt the dose and may recommend that your daily dose of REVOLADE be increased or decreased.
Please expect that at the beginning of therapy your platelet count and other routine blood parameters will need to be monitored frequently. Your doctor will also carry out blood tests to check your liver function before and during treatment with REVOLADE.
How to take it
Swallow REVOLADE with a glass of water.
If you take more REVOLADE than you should (Overdose)
Immediately telephone your doctor or the Poisons Information Centre (In Australia call 13 11 26. In New Zealand call 0800 POISON or 0800 764 766) for advice, or go to Accident and Emergency at the nearest hospital, if you think that you or anyone else may have taken too much REVOLADE. Do this even if there are no signs of discomfort or poisoning. If you are not sure what to do, contact your doctor or pharmacist.
If you forget to take REVOLADE
Do not take a double dose to make up for a forgotten dose.
If you have any further questions on the use of REVOLADE, ask your doctor or pharmacist.
Once you have started taking REVOLADE
Do not stop taking REVOLADE before talking to your doctor or pharmacist.
After you have stopped taking REVOLADE, your bleeding symptoms may come back. Tell your doctor or pharmacist if you have any bleeding in the 4 weeks after you stop taking REVOLADE.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
WHAT ARE THE POSSIBLE SIDE-EFFECTS
Like all medicines, REVOLADE can cause side effects, although not everybody gets them.
The following side effects have been reported to be associated with treatment with REVOLADE in patients with ITP.
Very common side effects
These may affect more than 1 in 10 people treated with REVOLADE.
Nausea and diarrhoea
Common side effects
These may affect up to 1 in 10 people treated with REVOLADE.
Increase of liver enzymes called aspartate and alanine transaminases,
Dry mouth
Vomiting
Alopecia (unusual hair loss or thinning)
Rash
Back pain
Musculoskeletal chest pain, musculoskeletal pain (pain that affects muscles and tendons along with bones)
Myalgia (aching muscles)Pharyngitis (sore throat and discomfort when swallowing)
Urinary tract infection (infection in the system that produces urine).
Other side effects
Other side effects have occurred during further studies with REVOLADE
Rare side effects (these may affect up to 1 in 1,000 people):
Clots in small blood vessels, which may harm organs such as the kidneys (Microangiopathy associated with renal impairment)
The following side effects have been reported to be associated with treatment with REVOLADE in combination with peginterferon and ribavirin in patients with HCV.
Very common side effects
These may affect more than 1 in 10 people treated with REVOLADE and antiviral agents.
Fever
Fatigue
Chills
Headache
Cough
Nausea
Diarrhoea
Unusual hair loss or thinning
Muscle pain
Itching
Feeling weak
Difficulty sleeping
Loss of appetite
Flu-like symptoms
Oedema peripheral (swelling of the hands, ankles or feet)
Very common side effects that may show up in blood tests
Anaemia (reduced number of red blood cells)
Common side effects that may show up in blood tests
These may affect up to 1 in 10 people treated with REVOLADE and antiviral agents.
Increase in bilirubin (a substance produced by the liver)
Tell your doctor immediately if you get any of these symptoms. These symptoms may persist after you stop taking REVOLADE.
Liver problems
REVOLADE may damage your liver and cause serious, even life threatening, illness. You must have blood tests to check your liver before you start taking REVOLADE and during treatment. When you are given certain antiviral treatments together with REVOLADE for the treatment of thrombocytopenia due to hepatitis C virus (HCV) infections some liver problems can get worse.
Your doctor will order these blood tests and any other tests required. In some cases REVOLADE treatment may need to be stopped. Tell your doctor right away if you have any of these signs and symptoms of liver problems:
jaundice (yellowing of the skin or the whites of the eyes)
unusual darkening of the urine
unusual tiredness
right upper stomach area pain
Bleeding after you stop treatment
When you stop taking REVOLADE, your blood platelet count will drop back down to what it was before you started taking REVOLADE. These effects are most likely to happen within 4 weeks after you stop taking REVOLADE. The lower platelet counts may increase your risk of bleeding. Your doctor will check your platelet counts for at least 4 weeks after you stop taking REVOLADE. Tell your doctor or pharmacist if you have any bruising or bleeding after you stop taking REVOLADE.
You may have problems with your bone marrow
People with the disease for which you are being treated may have problems with their bone marrow. Medicines like REVOLADE could make this problem worse. Your doctor may also carry out tests to check your bone marrow during treatment with REVOLADE.
High platelet counts and higher chance for blood clots
You have a higher chance of getting a blood clot if your platelet count is too high during treatment with REVOLADE, but blood clots can occur with normal or even low platelet counts. If you have cirrhosis of the liver you are at risk of a blood clot in a blood vessel that feeds your liver (portal vein thrombosis). You may have severe complications from some forms of blood clots, such as clots that travel to the lungs or that cause heart attacks or strokes. Your doctor will check your blood platelet counts, and change your dose or stop REVOLADE if your platelet counts get too high. Tell your doctor right away if you have signs and symptoms of a blood clot in the leg, such as swelling or pain/tenderness of one leg.
If any of the side effects listed in this leaflet get serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
Tell your doctor or pharmacist if you have any bleeding in the 4 weeks after you stop taking REVOLADE.
STORAGE
Do not store above 30°C.
Keep out of the reach and sight of children.
Do not use REVOLADE after the expiry date which is stated on the carton.
Disposal
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required.
PRODUCT DESCRIPTION
What REVOLADE looks like
REVOLADE is presented in packs of 28 tablets.
25 mg tablets
REVOLADE film-coated tablets are round, biconvex, white, debossed with 'GS NX3' and '25' on one side.
50 mg tablets
REVOLADE film-coated tablets are round, biconvex, brown, debossed with 'GS UFU' and '50' on one side.
75 mg tablets
REVOLADE film-coated tablets are round, biconvex, pink, debossed with 'GS FSS' and '75' on one side.
Ingredients
REVOLADE contains the active ingredient eltrombopag olamine.
REVOLADE also contains hypromellose, macrogol 400, magnesium stearate, mannitol, cellulose - microcrystalline, povidone, sodium starch glycollate, titanium dioxide (E171).
25 mg
The 25 mg tablet also contains polysorbate 80.
50 mg
The 50 mg tablet also contains iron oxide red CI77491 (E172) and iron oxide yellow CI77492 (E172).
75 mg
The 75 mg tablet also contains iron oxide black CI77499 and oxide red CI77491 (E172).
SUPPLIER
Your REVOLADE is supplied by:
GlaxoSmithKline Australia Ltd
Level 4, 436 Johnston St,
Abbotsford, Victoria, 3067
AUSTRALIA.
or
GlaxoSmithKline NZ Ltd
Private Bag 106600
Downtown
Auckland 1143
NEW ZEALAND
WHERE TO GO FOR FURTHER INFORMATION
Pharmaceutical companies are not in a position to give people an individual diagnosis or medical advice. Your doctor or pharmacist is the best person to give you the individual advice you need.
In Australia: For more information on Revolade please go to www.gsk.com.au/revolade or your doctor or pharmacist.
In New Zealand: For more information on Revolade please go to your doctor or pharmacist.
This leaflet was prepared on 28 July 2014.
The information provided applies only to REVOLADE®.
REVOLADE is a registered trade mark of the GlaxoSmithKline Group of Companies.
25 mg tablets AUST R 158419
50 mg tablets AUST R 158356
75 mg tablets AUST R 200121
© 2013 GlaxoSmithKline Australia
用药温馨提示:当您服用此药物时,需定期接受医疗专业人士的检查,以便随时针对其药效、副作用等情况进行监测。本网站所包含的信息旨在为患者提供帮助,不能代替医学建议和治疗。
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 艾曲波帕_REVOLADE_艾曲波帕片Eltrombopag说明书
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 艾曲波帕_REVOLADE_艾曲波帕片Eltrombopag说明书


