替西白介素重组冻干粉注射剂(Teceleukin)说明书
产地国家:日本 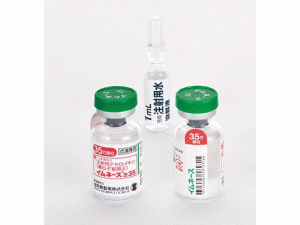 处 方 药:是
所属类别:35万单位/瓶 1瓶
包装规格:35万单位/瓶 1瓶
计价单位:套
生产厂家中文参考译名:盐野义制药
生产厂家英文名:Shionogi
原产地英文商品名:IMUNACE(イムネース注)35units/vial 1bottle(1mL of additional water for injection)/KIT
原产地英文药品名:Teceleukin(Genetical recombination)
中文参考商品译名:IMUNACE(イムネース注)35万单位/瓶 1瓶(附加注射用水1mL中)/套
中文参考药品译名:替西白介素基因重组
处 方 药:是
所属类别:35万单位/瓶 1瓶
包装规格:35万单位/瓶 1瓶
计价单位:套
生产厂家中文参考译名:盐野义制药
生产厂家英文名:Shionogi
原产地英文商品名:IMUNACE(イムネース注)35units/vial 1bottle(1mL of additional water for injection)/KIT
原产地英文药品名:Teceleukin(Genetical recombination)
中文参考商品译名:IMUNACE(イムネース注)35万单位/瓶 1瓶(附加注射用水1mL中)/套
中文参考药品译名:替西白介素基因重组
 处 方 药:是
所属类别:35万单位/瓶 1瓶
包装规格:35万单位/瓶 1瓶
计价单位:套
生产厂家中文参考译名:盐野义制药
生产厂家英文名:Shionogi
原产地英文商品名:IMUNACE(イムネース注)35units/vial 1bottle(1mL of additional water for injection)/KIT
原产地英文药品名:Teceleukin(Genetical recombination)
中文参考商品译名:IMUNACE(イムネース注)35万单位/瓶 1瓶(附加注射用水1mL中)/套
中文参考药品译名:替西白介素基因重组
处 方 药:是
所属类别:35万单位/瓶 1瓶
包装规格:35万单位/瓶 1瓶
计价单位:套
生产厂家中文参考译名:盐野义制药
生产厂家英文名:Shionogi
原产地英文商品名:IMUNACE(イムネース注)35units/vial 1bottle(1mL of additional water for injection)/KIT
原产地英文药品名:Teceleukin(Genetical recombination)
中文参考商品译名:IMUNACE(イムネース注)35万单位/瓶 1瓶(附加注射用水1mL中)/套
中文参考药品译名:替西白介素基因重组
简介
部份中文替西白介素基因重组处方资料(仅供参考) 英文名:Teceleukin 商品名:Imunace 35 中文名:替西白介素基因重组 生产商:盐野义制药 イムネース注35 药物类别名称:转基因白细胞介素-2配方 批准日期:1992年5月 欧文商標名:Imunace35 一般的名称 テセロイキン(遺伝子組換え)(JAN)[日局] Teceleukin(Genetical Recombination) 略号:rIL-2 分子式:C698H1127N179O204S8 分子量:15547.01 本質:转基因人类白细胞介素-2是一种由134种氨基酸残留物组成的蛋白质,甲氧丁与N-终点线结合。 性状 它是无色净度的液体。 药用药理学 1. 药理作用 (1) 抗肿瘤作用(体外) 6健康成人(5男1女)在培养72小时后,通过向外周血淋巴细胞中加入70个单位/mL获得,诱导3系人肾癌培养细胞具有很强的细胞毒性活性。 然而,对正常细胞(ConA刺激的人类正常淋巴细胞)的影响没有显示。 (2) 抗肿瘤作用(体内) 14 显示生存和转移抑制作用的延伸对Renca(自发小鼠肾癌)。 此外,对化学致癌性小鼠肾癌有显著的转移抑制作用。 2. 作用机制 它主要与T细胞和NK细胞结合,通过激活它们,它诱导具有高细胞毒性的杀伤细胞损害肿瘤。它也结合到B细胞和巨噬细胞,激活免疫。 适应症 1. 血管造影肉瘤 2. 肾癌 用量用法 1.血管造影肉瘤 溶解在盐水溶液或5%葡萄糖注射液或类似,通常,70万单位每天在成人,静脉注射每天分为1-2次,每天。 应该注意的是,最大剂量为每天140万单位,虽然年龄和适当的增加或减少取决于症状。 2.肾癌 溶解在盐水溶液或5%葡萄糖注射液或类似,通常,70万单位每天在成人,静脉注射每天分为1-2次,每天。 应该注意的是,最大剂量为每天210万单位,虽然年龄和适当的增加或减少取决于症状。 要小心,因为肝功能测试值异常和液体保留更可能通过增加量来表示。 参考:注射的制备方法 1.为每瓶每日注射添加1mL水(350,000单位Tessereokin),并溶解。 2.输注除200~500mL如盐水溶液或5%葡萄糖注射液等外,一旦剂量静脉注射。 临床表现 1. 血管造影肉瘤 批准时一般临床试验的疗效评价指标为11例,性能效率为36.4%(CR1和PR3例)。 表2见临床结果(血管肉瘤) 2. 肾癌 批准时一般临床试验的疗效评价指标为119例,性能效率为14.3%(CR3和PR14例)。其中,干扰素(IFN)无效66例,性能效率为13.6%(CR1和PR8例)。 表3见临床结果(肾癌) 包装规格 注 35:1瓶:附着在水1mL上,用于每日注射。 制造和销售 盐野义制药有限公司 注:以上中文处方资料不够完整,使用者以原处方资料为准。英文版说明书
IMUNACE35 for Injection(イムネース注35) Brand name: IMUNACE35 for Injection Active ingredient: Teceleukin (Genetical recombination) Dosage form: injection Print on wrapping: Effects of this medicine This medicine improves immunity by activating lymphocytes, and it damages cancer cells via immune reactions. Furthermore, it shows an inhibitory effect against metastasis of cancer. It is usually used for the treatment of angiosarcoma and kidney cancer. Before using this medicine, be sure to tell your doctor and pharmacist •If you have previously experienced any allergic reactions (itch, rash, etc.) to any medicines and vaccines. •If you are pregnant or breastfeeding. •If you are taking any other medicinal products. (Some medicines may interact to enhance or diminish medicinal effects. Beware of over-the-counter medicines and dietary supplements as well as other prescription medicines.) Dosing schedule (How to take this medicine) •Your dosing schedule prescribed by your doctor is<> •In general, for adults, this medicine is administered by intravenous drip infusion daily in 1 to 2 divided doses on consecutive days. •The schedule for use is determined according to your symptoms. Consult with your doctor about your concrete schedule for use. Precautions while taking this medicine •If you are breastfeeding, avoid breastfeeding while using this medicine. Possible adverse reactions to this medicine The most commonly reported adverse reactions include fever, chill, shiver, malaise, loss of appetite, nausea, vomiting, headache, diarrhea, weight gain, edema, water retention, decreased urine output, retention of pleural effusion (dry cough, breathlessness, chest pain), breathing difficulty, deperession, hypotension, irregular heartbeat, palpitation, frequent pulse and cold sense in lower limbs. If any of these symptoms occur, consult with your doctor or pharmacist. The symptoms described below are rarely seen as initial symptoms of the adverse reactions indicated in brackets. If any of these symptoms occur, stop taking this medicine and see your doctor immediately. •weight gain, swelling, decreased urine output [retention of body fluid] •swelling, shortness of breath, cough [congestive heart failure] •feeling depressed and unmotivated, sleeplessness, malaise, suicide attempt [depression, suicide attempt] •infection by bacteria, fever, cough [(in case of using a large amount of it) induced infection, exacerbation of infection] •malaise, yellowness in skin and in the white of eyes, thirstiness, increased urine output [autoimmune phenomenon] The above symptoms do not describe all the adverse reactions to this medicine. Consult with your doctor or pharmacist if you notice any symptoms of concern other than those listed above. Shionogi & Co., Ltd.Injection Published: 2/2014 The information on this sheet is based on approvals granted by the Japanese regulatory authority. Approval details may vary by country. Medicines have adverse reactions (risks) as well as efficacies (benefits). It is important to minimize adverse reactions and maximize efficacy. To obtain a better therapeutic response, patients should understand their medication and cooperate with the treatment.用药温馨提示:当您服用此药物时,需定期接受医疗专业人士的检查,以便随时针对其药效、副作用等情况进行监测。本网站所包含的信息旨在为患者提供帮助,不能代替医学建议和治疗。
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 替西白介素重组冻干粉注射剂(Teceleukin)说明书
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 替西白介素重组冻干粉注射剂(Teceleukin)说明书