托吡酯片说明书_TOPINA_Topiramate
 药店国别:
产地国家:日本
处 方 药:是
所属类别:50毫克/片 100片/盒
包装规格:50毫克/片 100片/盒
计价单位:盒
生产厂家中文参考译名:协和发酵麒麟
生产厂家英文名:Kyowa Hakko Kirin Co.Ltd
原产地英文商品名:TOPINA 50mg/Tablets 100Tablets/box
原产地英文药品名:Topiramate
中文参考商品译名:TOPINA 50毫克/片 100片/盒
中文参考药品译名:托吡酯
简介:
部份中文托吡酯处方资料(仅供参考)
英文名:Topiramate
商标名:TOPINA Tablets
中文名:托吡酯
剂 型:片剂
生产商:协和发酵麒麟
药物分类名称:抗癫痫药
商標名:
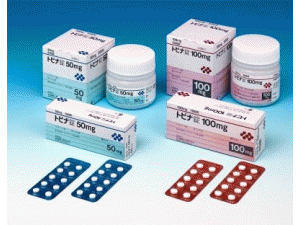
TOPINA Tablets 25mg
TOPINA Tablets 50mg
TOPINA Tablets 100mg
一般名:Topiramate
化学名:(-)-2, 3:4, 5-Di-O-isopropylidene-β-D-fructopyranose sulfamate
分子式:C12H21NO8S=339.36
性状:
它是白色水晶,没有异味,味道很苦。
可溶性:
它易溶于甲醇或乙醇(99.5),难以溶于水。
分配系数:
log P'OCT = 0.59
(测量方法:烧瓶方法正辛醇/ pH 7缓冲溶液)
药用药理学:
1.药理作用
抑制最大电击惊厥(老鼠)。
抑制部分癫痫模型(大鼠)的点燃痉挛。
在遗传性癫痫模型中抑制强直性痉挛和癫痫发作(自发性癫痫大鼠)和听源性癫痫发作(DBA /2小鼠)。
抑制短暂的全脑缺血和出生后缺氧诱发的惊厥(大鼠)。
2.作用机制
该药抑制持续去极化脉冲引起的频繁点火,抑制L型钙电流,抑制红藻氨酸诱导的内向电流,通过GABAA受体促进氯离子通过GABAA受体流入,以及人体碳酸酐脱水 观察到酶(II型和IV型)的抑制。
基于这些事实,该产品的抗癫痫作用是电压依赖性钠通道抑制作用,电压依赖性L型钙通道抑制作用,AMPA(α-氨基-3-羟基-5-甲基异恶唑-4-丙酸)推测其基于红藻氨酸型谷氨酸受体功能抑制作用,GABA存在下的GABAA受体功能增强作用和碳酸酐酶抑制作用。
适应症:
联合用抗癫痫药物治疗癫痫患者部分性癫痫发作(包括继发性全身性癫痫发作)对其他抗癫痫药物无效
用法用量:
成人:
通常,成人应该开始口服50毫克,每天一次或每天两次作为托吡酯。此后,它以1周或更长的间隔逐渐增加,并且以两个分剂量口服给予200-400mg的日剂量作为维持剂量。
此外,根据症状,它会适当增加或减少,但最大日剂量应为600毫克。
儿童:
对于2岁及以上的儿童,通常每天口服1mg/kg作为托吡酯,并以2周或更长的间隔每日增加至2 mg/kg。 之后,以2周或更长的间隔逐渐增加2mg/kg或更少的日剂量,并以每天6mg/kg的维持剂量口服给药。 根据症状,它会相应增加或减少,但最大日剂量应高达9毫克/千克或600毫克,以较低者为准。 两者均应每天两次口服给药。
包装:
片剂
25毫克:[PTP] 100片(10片×10)[瓶装] 500片
50毫克:[PTP] 100片(10片×10)[瓶装] 500片
100毫克:[PTP] 100片(10片×10)[瓶装] 500片
制造供应商:
协和发酵麒麟有限公司
New release of anti epileptic drug "Topina ® tablets 50 mg, 100 mg"
September 26, 2007
Kyowa Hakko (President: Chiyoda-ku, Tokyo: President: Joe Matsuda) began selling on September 26, 2007 on "Topina ® Tablets 50 mg" and "Topina ® Tablets 100 mg" (Generic name: Topiramate) I will let you know.
Topiramate is an antiepileptic drug created in the United States, was approved in the United Kingdom in 1995 and then in the United States in 1996 and is currently approved in more than 100 countries.
In Japan, our company has undergone clinical development and "efficacy of combination therapy with antiepileptic drugs for partial seizures (including secondary generalized seizures) of epilepsy patients who are not adequately effective with other antiepileptic drugs" · Acquired approval on July 31st this year with effect, listed on the drug price standard on September 21st.
Since 1975, we have sought to market antiepileptic drugs "Depapen ®", and we have various types of tablets (syrup, fine grain, sustained release formulation), mainly contributing to the treatment of general epilepsy, We believe that by adding "Topina ® tablets" which have an effect of suppressing the seizure of partial epilepsy of intractable epilepsy, we can contribute and contribute to the field of epilepsy treatment even more.
Product summary of Anti epileptic drug Topin® tablet 50mg, same 100mg Product name "Topina ® tablet 50mg", "Topina ® tablet 100mg" (common name: Topiramate/ Topiramate)
Indications/Combination therapy with antiepileptic drugs for partial seizures (including secondary generalized seizures) of epilepsy patients who are not adequately effective with other antiepileptic drugs
Dosage/dosage Usually for adults start as a single dose of 50 mg once daily or twice daily orally as topiramate. Thereafter, it gradually increases at intervals of 1 week or more, and the daily dose of 200 to 400 mg is orally administered as a maintenance dose in two divided doses. Incidentally, depending on the symptoms, it will be increased or decreased appropriately, but the maximum daily dose should be 600 mg.
Main features Anti-convulsant action with a wide range of action mechanisms including AMPA/kainate type glutamate receptor function inhibitory action etc.
Side effects The side effects in domestic II/III phase and long-term administration tests are recognized in 228 cases (75.2%) out of 303 cases. The main side effects were somnolence 90 cases (29.7%), weight loss 75 cases (24.8%), floating dizziness 44 cases (14.5%), anorexia and bulimia syndrome 32 cases (10.6%),
Approval Date of acquisition July 31, 2007
Drug price standard
Listing date September 21, 2007
Sales start date September 26, 2007
Manufacturer Distributor Kyowa Hakko Kogyo Co.Ltd.
药店国别:
产地国家:日本
处 方 药:是
所属类别:50毫克/片 100片/盒
包装规格:50毫克/片 100片/盒
计价单位:盒
生产厂家中文参考译名:协和发酵麒麟
生产厂家英文名:Kyowa Hakko Kirin Co.Ltd
原产地英文商品名:TOPINA 50mg/Tablets 100Tablets/box
原产地英文药品名:Topiramate
中文参考商品译名:TOPINA 50毫克/片 100片/盒
中文参考药品译名:托吡酯
简介:
部份中文托吡酯处方资料(仅供参考)
英文名:Topiramate
商标名:TOPINA Tablets
中文名:托吡酯
剂 型:片剂
生产商:协和发酵麒麟
药物分类名称:抗癫痫药
商標名:
TOPINA Tablets 25mg
TOPINA Tablets 50mg
TOPINA Tablets 100mg
一般名:Topiramate
化学名:(-)-2, 3:4, 5-Di-O-isopropylidene-β-D-fructopyranose sulfamate
分子式:C12H21NO8S=339.36
性状:
它是白色水晶,没有异味,味道很苦。
可溶性:
它易溶于甲醇或乙醇(99.5),难以溶于水。
分配系数:
log P'OCT = 0.59
(测量方法:烧瓶方法正辛醇/ pH 7缓冲溶液)
药用药理学:
1.药理作用
抑制最大电击惊厥(老鼠)。
抑制部分癫痫模型(大鼠)的点燃痉挛。
在遗传性癫痫模型中抑制强直性痉挛和癫痫发作(自发性癫痫大鼠)和听源性癫痫发作(DBA /2小鼠)。
抑制短暂的全脑缺血和出生后缺氧诱发的惊厥(大鼠)。
2.作用机制
该药抑制持续去极化脉冲引起的频繁点火,抑制L型钙电流,抑制红藻氨酸诱导的内向电流,通过GABAA受体促进氯离子通过GABAA受体流入,以及人体碳酸酐脱水 观察到酶(II型和IV型)的抑制。
基于这些事实,该产品的抗癫痫作用是电压依赖性钠通道抑制作用,电压依赖性L型钙通道抑制作用,AMPA(α-氨基-3-羟基-5-甲基异恶唑-4-丙酸)推测其基于红藻氨酸型谷氨酸受体功能抑制作用,GABA存在下的GABAA受体功能增强作用和碳酸酐酶抑制作用。
适应症:
联合用抗癫痫药物治疗癫痫患者部分性癫痫发作(包括继发性全身性癫痫发作)对其他抗癫痫药物无效
用法用量:
成人:
通常,成人应该开始口服50毫克,每天一次或每天两次作为托吡酯。此后,它以1周或更长的间隔逐渐增加,并且以两个分剂量口服给予200-400mg的日剂量作为维持剂量。
此外,根据症状,它会适当增加或减少,但最大日剂量应为600毫克。
儿童:
对于2岁及以上的儿童,通常每天口服1mg/kg作为托吡酯,并以2周或更长的间隔每日增加至2 mg/kg。 之后,以2周或更长的间隔逐渐增加2mg/kg或更少的日剂量,并以每天6mg/kg的维持剂量口服给药。 根据症状,它会相应增加或减少,但最大日剂量应高达9毫克/千克或600毫克,以较低者为准。 两者均应每天两次口服给药。
包装:
片剂
25毫克:[PTP] 100片(10片×10)[瓶装] 500片
50毫克:[PTP] 100片(10片×10)[瓶装] 500片
100毫克:[PTP] 100片(10片×10)[瓶装] 500片
制造供应商:
协和发酵麒麟有限公司
New release of anti epileptic drug "Topina ® tablets 50 mg, 100 mg"
September 26, 2007
Kyowa Hakko (President: Chiyoda-ku, Tokyo: President: Joe Matsuda) began selling on September 26, 2007 on "Topina ® Tablets 50 mg" and "Topina ® Tablets 100 mg" (Generic name: Topiramate) I will let you know.
Topiramate is an antiepileptic drug created in the United States, was approved in the United Kingdom in 1995 and then in the United States in 1996 and is currently approved in more than 100 countries.
In Japan, our company has undergone clinical development and "efficacy of combination therapy with antiepileptic drugs for partial seizures (including secondary generalized seizures) of epilepsy patients who are not adequately effective with other antiepileptic drugs" · Acquired approval on July 31st this year with effect, listed on the drug price standard on September 21st.
Since 1975, we have sought to market antiepileptic drugs "Depapen ®", and we have various types of tablets (syrup, fine grain, sustained release formulation), mainly contributing to the treatment of general epilepsy, We believe that by adding "Topina ® tablets" which have an effect of suppressing the seizure of partial epilepsy of intractable epilepsy, we can contribute and contribute to the field of epilepsy treatment even more.
Product summary of Anti epileptic drug Topin® tablet 50mg, same 100mg Product name "Topina ® tablet 50mg", "Topina ® tablet 100mg" (common name: Topiramate/ Topiramate)
Indications/Combination therapy with antiepileptic drugs for partial seizures (including secondary generalized seizures) of epilepsy patients who are not adequately effective with other antiepileptic drugs
Dosage/dosage Usually for adults start as a single dose of 50 mg once daily or twice daily orally as topiramate. Thereafter, it gradually increases at intervals of 1 week or more, and the daily dose of 200 to 400 mg is orally administered as a maintenance dose in two divided doses. Incidentally, depending on the symptoms, it will be increased or decreased appropriately, but the maximum daily dose should be 600 mg.
Main features Anti-convulsant action with a wide range of action mechanisms including AMPA/kainate type glutamate receptor function inhibitory action etc.
Side effects The side effects in domestic II/III phase and long-term administration tests are recognized in 228 cases (75.2%) out of 303 cases. The main side effects were somnolence 90 cases (29.7%), weight loss 75 cases (24.8%), floating dizziness 44 cases (14.5%), anorexia and bulimia syndrome 32 cases (10.6%),
Approval Date of acquisition July 31, 2007
Drug price standard
Listing date September 21, 2007
Sales start date September 26, 2007
Manufacturer Distributor Kyowa Hakko Kogyo Co.Ltd.
用药温馨提示:当您服用此药物时,需定期接受医疗专业人士的检查,以便随时针对其药效、副作用等情况进行监测。本网站所包含的信息旨在为患者提供帮助,不能代替医学建议和治疗。
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 托吡酯片说明书_TOPINA_Topiramate
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 托吡酯片说明书_TOPINA_Topiramate