环孢素A胶囊CICLOSPORIN Capsules 10mg说明书
[caption id="attachment_29241" align="alignleft" width="300"]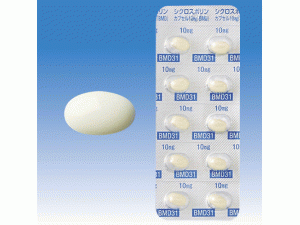 环孢素A胶囊CICLOSPORIN Capsules 10mg[/caption]
药店国别:
产地国家:日本
处 方 药:是
所属类别:10毫克/胶囊 100胶囊/盒
包装规格:10毫克/胶囊 100胶囊/盒
计价单位:盒
生产厂家中文参考译名:富士药业
生产厂家英文名:Fuji Pharmaceutical
原产地英文商品名:CICLOSPORIN10mg/Capsules 100Capsules
原产地英文药品名:Ciclosporin
中文参考商品译名:CICLOSPORIN胶囊10毫克/胶囊 100胶囊/盒
中文参考药品译名:环孢素
简介:
部份中文環孢素A處方資料(僅供參考)
药物分类名称:免疫抑制剂
批准日期:2011年6月
欧文商标名:CICLOSPORIN Capsules
一般名:シクロスポリン (Ciclosporin)
別名:サイクロスポリンA
化学名:cyclo {-[(2S ,3R ,4R ,6E )- 3-Hydroxy-4-methyl-2-methylaminooct-6-enoyl]-L-2-aminobutanoyl-N -methylglycyl-N -methyl-L-leucyl-L-valyl-N-methyl-L-leucyl-L-alanyl-D-alanyl-N-methyl-L-leucyl-N-methyl-L-leucyl-N -methyl-L-valyl-}
分子式:C62H111N11O12
分子量:1202.61
性状:
它是一种白色的粉末。
它非常易溶于乙腈,甲醇或乙醇(95),易溶于乙醚,难溶于水。
处理注意事项:
“稳定性测试”
(1)环孢菌素胶囊10 mg“BMD”环孢菌素胶囊10 mg“BMD”在正常市场循环下使用最终包装产品“加速试验(40°C,相对湿度75%,6个月”)稳定3年,据推测
(2)环孢菌素胶囊25mg“BMD”作为使用最终包装产品的长期保存试验(室温保存3年)的结果,外观和含量等在规格范围内,环孢菌素胶囊25mg“BMD”经证实,在经销3年后稳定。
(3)环孢菌素胶囊50mg“BMD”使用最终包装产品,外观和含量的长期储存试验(室温保存3年)的结果在规格范围内,环孢素胶囊50mg“BMD”经证实,在经销3年后稳定。
由于胶囊可能由于吸湿而变软,因此请将它们保持在PTP包装中,直到服用。
适应病症:
在以下器官移植中抑制排斥反应
1.肾移植,肝移植,心脏移植,肺移植,胰移植,小肠移植
2.在骨髓移植中抑制排斥反应和移植物抗宿主病
3.白塞病(在眼部症状存在时)和其他非感染性葡萄膜炎(在现有治疗中有效,活跃部位具有降低非感染性葡萄膜炎视力风险)有限的)
4.寻常型银屑病(皮肤发炎超过全身的30%或难治性的情况),脓疱性牛皮癣,银屑病性红皮病,关节病牛皮癣。
5.再生障碍性贫血(严重),成红细胞。
6.肾病综合征(频繁复发或抵抗类固醇的病例)
7.系统型重症肌无力(在胸腺切除术后由于副作用引起的类固醇药物无效或难治)
8.特应性皮炎(无法获得现有治疗效果的患者)
用法与用量:
1.在肾移植的情况下
通常,作为移植前1天的环孢菌素,日剂量为9〜12mg/kg,每天口服两次,然后每天减少2mg / kg。 标准维持剂量为每日4〜6 mg / kg,但应根据症状进行增减。
2.在肝移植的情况下
通常,作为移植前1天的环孢菌素,每日口服两次给药,每日剂量为14〜16mg / kg。 此后,剂量逐渐降低,维持剂量设定为5〜10mg / kg /日标准,但根据症状适当增减。
3.在心脏移植的情况下,肺移植,胰腺移植
通常,从移植前1天起,每天口服两次,每天口服10〜15mg/kg,作为环孢菌素。 此后,剂量逐渐降低,维持剂量设定为2〜6mg /kg/日,作为标准,但根据症状适当增减。
4.在小肠移植的情况下
通常,环孢菌素以14至16mg/kg的日剂量口服给药,每天分成两次。 此后,剂量逐渐降低,维持剂量设定为5〜10mg / kg /日标准,但根据症状适当增减。 然而,从正常移植前1天开始注射环孢菌素后,可以口服给药,尽快切换口服。
5.在骨髓移植的情况下
通常,移植前1天,日剂量为6〜12mg/kg,每日口服2次,作为环孢素,持续3〜6个月,然后逐渐减量停止。
6.在贝切特病和其他非传染性葡萄膜炎的情况下
通常,口服给药是以每日两次5mg/kg的日剂量作为环孢菌素分开开始,然后每月每天减少或增加1〜2mg / kg。 维持剂量为每日3〜5mg / kg,作为标准,但应按适当的症状增加或减少。
7.在牛皮癣的情况下
通常,每日剂量为5mg/kg以两个分剂量施用。 如果观察到效果,每月减少1毫克/公斤/天,维持剂量为每日3毫克/公斤。 此外,应该根据症状增加或减少。
8.在再生障碍性贫血的情况下
通常,每天两次,以6mg/kg的日剂量口服给予环孢菌素。 此外,应该根据症状增加或减少。 此外,由于具有短的疾病持续时间的患者可能具有更好的治疗效果,因此期望将疾病持续时间少于6个月的患者作为指导。
9.肾病综合征:
通常,口服以下剂量作为环孢菌素分成一天两次。 此外,应该根据症状增加或减少。
(1) 频繁的复发病例
对于成年人,施用1.5mg/kg的日剂量。 在儿童的情况下,每天服用2.5mg/kg。
(2) 显示对类固醇抵抗的病例
对于成年人,每日给药3mg/kg。 在儿童的情况下,每天服用5毫克/千克。
10.在系统性重症肌无力的情况下
通常每天口服5毫克/千克作为环孢素分成两天。 如果观察到效果,逐渐降低剂量,维持3 mg/kg为标准。 此外,应该根据症状增加或减少。
11.在特应性皮炎的情况下
通常,成人每天口服3mg/kg,因为环孢菌素每天分成两次。 此外,应该根据症状增加或减少,但每天不应超过5毫克/千克
包装规格:
环孢菌素胶囊
10mg“BMD”100粒(10粒×10; PTP)
25 mg“BMD”100粒(10粒×10; PTP)
50 mg“BMD”100粒(10粒×10; PTP)
制造厂商:富士药业
Cyclosporine capsule 10 mg
Indication
① Suppression of rejection in kidney transplant
② Suppression of rejection in liver transplantation
③ Suppression of rejection in heart transplantation, lung transplantation and pancreatic transplantation
④ Suppression of rejection in small bowel transplantation
⑤ Suppression of rejection and graft-versus-host disease in bone marrow transplantation
⑥ Behcet's disease (in the presence of ocular symptoms) and other non-infectious uveitis (in non-infectious uveitis in the middle or the rear of the activity which is ineffective in existing treatments, Limited)
⑦ Vulgaris psoriasis (in cases where the skin eruption is over 30% of the whole body or if it is refractory), pustular psoriasis, psoriatic erythrosis, arthropathic psoriasis
⑧ aplastic anemia (severe), erythroblasts
⑨ Nephrotic syndrome (in cases of frequent recurrence or resistance to steroids)
⑩ Whole body type myasthenia gravis (in cases where administration of steroid drug is ineffective or difficult due to side effects in treatment after thymectomy)
⑪ Atopic dermatitis (a patient who can not obtain sufficient effect by the existing treatment
Usage · capacity
① From 1 day before transplantation, daily dose of 9 to 12 mg / kg is divided into two doses twice daily, and then the dose is reduced by 2 mg / kg daily. Maintenance amount: daily dose 4 to 6 mg / kg
② From 1 day before transplantation, 14-16 mg/kg per day is divided into two doses twice a day, then gradually reduced, maintenance dose: 5 to 10 mg / kg daily
③ From 1 day before the transplantation, 10 to 15 mg/kg per day is distributed twice a day, then gradually reduced, maintenance dose: 2 to 6 mg / kg daily
④ Daily dose of 14 to 16 mg/kg divided into twice daily. Starting with cyclosporine injection starting from 1 day before usual transplantation, administration is switched to oral administration as soon as possible after oral administration becomes possible
⑤ From 6 days before transplantation, 6 to 12 mg/kg is administered orally twice a day, continued for 3 to 6 months, then gradually decreased and discontinued
⑥ Start daily dose 5 mg/kg in divided doses twice a day, then reduce or increase 1 to 2 mg / kg per day every other month. Maintenance dose: daily dose 3 to 5 mg / kg
⑦ Daily dose 5 mg/kg divided into two doses. When effect is observed 1 mg / kg per day is reduced every 1 month, maintenance amount: 3 mg / kg per day
⑧ Daily dose of 6 mg/kg divided into twice daily. It is advisable to target patients with disease duration of less than 6 months
⑨ Apply the next dose to twice daily. Frequent recurrent cases: daily dose 1.5 mg / kg, children; daily dose 2.5 mg / kg. Cases showing resistance to steroids: 3 mg / kg daily, pediatric: 5 mg / kg daily
⑩ Daily dose of 5 mg/kg divided into twice daily. When effect was observed gradually decrease, keep: 3 mg / kg
⑪ Adult daily dose 3 mg / kg divided into twice daily, increasing or decreasing accordingly, not exceeding the daily dose of 5 mg / kg
Side effect
This drug has not conducted a survey that clarifies the frequency of occurrence of adverse reactions such as survey results.
1. Serious side effects (Frequency unknown)
1) Renal disorder: Renal function disorder is frequently observed as side effects of this drug, the main expression mechanism is thought to be due to dose-dependent renal vasoconstriction action, and usually recovered by weight loss or withdrawal [ BUN elevation, creatinine elevation showing renal blood flow reduction.
Usage notes
(Contraindications)
This drug ingredient hypersensitivity history history, pregnant women, potential for pregnancy, lactating women, tacrolimus (excluding topical preparations), pitavastatin, rosuvastatin, bosentan, aliskiren while taking colchicine in patients with liver / kidney disorder ) Neuro Behcet's Disease (Warning) This drug administration in organ transplantation should be performed under the guidance of a doctor who is familiar with immunosuppressive therapy and transplant patient management. In the case of atopic dermatitis, administration of this drug to a doctor who is familiar with the treatment of atopic dermatitis sufficiently explained the effectiveness and danger to the patient or its family in advance and confirmed that they understood Dosing should begin with. Since this drug is not biologically equivalent to Sandimmun (liquid for internal use or capsule) and its bioavailability is improved, when switching from Sandimmun to this drug, the concentration of cyclosporin in blood (AUC, Cmax ) To raise the side effects caused by the rise.
环孢素A胶囊CICLOSPORIN Capsules 10mg[/caption]
药店国别:
产地国家:日本
处 方 药:是
所属类别:10毫克/胶囊 100胶囊/盒
包装规格:10毫克/胶囊 100胶囊/盒
计价单位:盒
生产厂家中文参考译名:富士药业
生产厂家英文名:Fuji Pharmaceutical
原产地英文商品名:CICLOSPORIN10mg/Capsules 100Capsules
原产地英文药品名:Ciclosporin
中文参考商品译名:CICLOSPORIN胶囊10毫克/胶囊 100胶囊/盒
中文参考药品译名:环孢素
简介:
部份中文環孢素A處方資料(僅供參考)
药物分类名称:免疫抑制剂
批准日期:2011年6月
欧文商标名:CICLOSPORIN Capsules
一般名:シクロスポリン (Ciclosporin)
別名:サイクロスポリンA
化学名:cyclo {-[(2S ,3R ,4R ,6E )- 3-Hydroxy-4-methyl-2-methylaminooct-6-enoyl]-L-2-aminobutanoyl-N -methylglycyl-N -methyl-L-leucyl-L-valyl-N-methyl-L-leucyl-L-alanyl-D-alanyl-N-methyl-L-leucyl-N-methyl-L-leucyl-N -methyl-L-valyl-}
分子式:C62H111N11O12
分子量:1202.61
性状:
它是一种白色的粉末。
它非常易溶于乙腈,甲醇或乙醇(95),易溶于乙醚,难溶于水。
处理注意事项:
“稳定性测试”
(1)环孢菌素胶囊10 mg“BMD”环孢菌素胶囊10 mg“BMD”在正常市场循环下使用最终包装产品“加速试验(40°C,相对湿度75%,6个月”)稳定3年,据推测
(2)环孢菌素胶囊25mg“BMD”作为使用最终包装产品的长期保存试验(室温保存3年)的结果,外观和含量等在规格范围内,环孢菌素胶囊25mg“BMD”经证实,在经销3年后稳定。
(3)环孢菌素胶囊50mg“BMD”使用最终包装产品,外观和含量的长期储存试验(室温保存3年)的结果在规格范围内,环孢素胶囊50mg“BMD”经证实,在经销3年后稳定。
由于胶囊可能由于吸湿而变软,因此请将它们保持在PTP包装中,直到服用。
适应病症:
在以下器官移植中抑制排斥反应
1.肾移植,肝移植,心脏移植,肺移植,胰移植,小肠移植
2.在骨髓移植中抑制排斥反应和移植物抗宿主病
3.白塞病(在眼部症状存在时)和其他非感染性葡萄膜炎(在现有治疗中有效,活跃部位具有降低非感染性葡萄膜炎视力风险)有限的)
4.寻常型银屑病(皮肤发炎超过全身的30%或难治性的情况),脓疱性牛皮癣,银屑病性红皮病,关节病牛皮癣。
5.再生障碍性贫血(严重),成红细胞。
6.肾病综合征(频繁复发或抵抗类固醇的病例)
7.系统型重症肌无力(在胸腺切除术后由于副作用引起的类固醇药物无效或难治)
8.特应性皮炎(无法获得现有治疗效果的患者)
用法与用量:
1.在肾移植的情况下
通常,作为移植前1天的环孢菌素,日剂量为9〜12mg/kg,每天口服两次,然后每天减少2mg / kg。 标准维持剂量为每日4〜6 mg / kg,但应根据症状进行增减。
2.在肝移植的情况下
通常,作为移植前1天的环孢菌素,每日口服两次给药,每日剂量为14〜16mg / kg。 此后,剂量逐渐降低,维持剂量设定为5〜10mg / kg /日标准,但根据症状适当增减。
3.在心脏移植的情况下,肺移植,胰腺移植
通常,从移植前1天起,每天口服两次,每天口服10〜15mg/kg,作为环孢菌素。 此后,剂量逐渐降低,维持剂量设定为2〜6mg /kg/日,作为标准,但根据症状适当增减。
4.在小肠移植的情况下
通常,环孢菌素以14至16mg/kg的日剂量口服给药,每天分成两次。 此后,剂量逐渐降低,维持剂量设定为5〜10mg / kg /日标准,但根据症状适当增减。 然而,从正常移植前1天开始注射环孢菌素后,可以口服给药,尽快切换口服。
5.在骨髓移植的情况下
通常,移植前1天,日剂量为6〜12mg/kg,每日口服2次,作为环孢素,持续3〜6个月,然后逐渐减量停止。
6.在贝切特病和其他非传染性葡萄膜炎的情况下
通常,口服给药是以每日两次5mg/kg的日剂量作为环孢菌素分开开始,然后每月每天减少或增加1〜2mg / kg。 维持剂量为每日3〜5mg / kg,作为标准,但应按适当的症状增加或减少。
7.在牛皮癣的情况下
通常,每日剂量为5mg/kg以两个分剂量施用。 如果观察到效果,每月减少1毫克/公斤/天,维持剂量为每日3毫克/公斤。 此外,应该根据症状增加或减少。
8.在再生障碍性贫血的情况下
通常,每天两次,以6mg/kg的日剂量口服给予环孢菌素。 此外,应该根据症状增加或减少。 此外,由于具有短的疾病持续时间的患者可能具有更好的治疗效果,因此期望将疾病持续时间少于6个月的患者作为指导。
9.肾病综合征:
通常,口服以下剂量作为环孢菌素分成一天两次。 此外,应该根据症状增加或减少。
(1) 频繁的复发病例
对于成年人,施用1.5mg/kg的日剂量。 在儿童的情况下,每天服用2.5mg/kg。
(2) 显示对类固醇抵抗的病例
对于成年人,每日给药3mg/kg。 在儿童的情况下,每天服用5毫克/千克。
10.在系统性重症肌无力的情况下
通常每天口服5毫克/千克作为环孢素分成两天。 如果观察到效果,逐渐降低剂量,维持3 mg/kg为标准。 此外,应该根据症状增加或减少。
11.在特应性皮炎的情况下
通常,成人每天口服3mg/kg,因为环孢菌素每天分成两次。 此外,应该根据症状增加或减少,但每天不应超过5毫克/千克
包装规格:
环孢菌素胶囊
10mg“BMD”100粒(10粒×10; PTP)
25 mg“BMD”100粒(10粒×10; PTP)
50 mg“BMD”100粒(10粒×10; PTP)
制造厂商:富士药业
Cyclosporine capsule 10 mg
Indication
① Suppression of rejection in kidney transplant
② Suppression of rejection in liver transplantation
③ Suppression of rejection in heart transplantation, lung transplantation and pancreatic transplantation
④ Suppression of rejection in small bowel transplantation
⑤ Suppression of rejection and graft-versus-host disease in bone marrow transplantation
⑥ Behcet's disease (in the presence of ocular symptoms) and other non-infectious uveitis (in non-infectious uveitis in the middle or the rear of the activity which is ineffective in existing treatments, Limited)
⑦ Vulgaris psoriasis (in cases where the skin eruption is over 30% of the whole body or if it is refractory), pustular psoriasis, psoriatic erythrosis, arthropathic psoriasis
⑧ aplastic anemia (severe), erythroblasts
⑨ Nephrotic syndrome (in cases of frequent recurrence or resistance to steroids)
⑩ Whole body type myasthenia gravis (in cases where administration of steroid drug is ineffective or difficult due to side effects in treatment after thymectomy)
⑪ Atopic dermatitis (a patient who can not obtain sufficient effect by the existing treatment
Usage · capacity
① From 1 day before transplantation, daily dose of 9 to 12 mg / kg is divided into two doses twice daily, and then the dose is reduced by 2 mg / kg daily. Maintenance amount: daily dose 4 to 6 mg / kg
② From 1 day before transplantation, 14-16 mg/kg per day is divided into two doses twice a day, then gradually reduced, maintenance dose: 5 to 10 mg / kg daily
③ From 1 day before the transplantation, 10 to 15 mg/kg per day is distributed twice a day, then gradually reduced, maintenance dose: 2 to 6 mg / kg daily
④ Daily dose of 14 to 16 mg/kg divided into twice daily. Starting with cyclosporine injection starting from 1 day before usual transplantation, administration is switched to oral administration as soon as possible after oral administration becomes possible
⑤ From 6 days before transplantation, 6 to 12 mg/kg is administered orally twice a day, continued for 3 to 6 months, then gradually decreased and discontinued
⑥ Start daily dose 5 mg/kg in divided doses twice a day, then reduce or increase 1 to 2 mg / kg per day every other month. Maintenance dose: daily dose 3 to 5 mg / kg
⑦ Daily dose 5 mg/kg divided into two doses. When effect is observed 1 mg / kg per day is reduced every 1 month, maintenance amount: 3 mg / kg per day
⑧ Daily dose of 6 mg/kg divided into twice daily. It is advisable to target patients with disease duration of less than 6 months
⑨ Apply the next dose to twice daily. Frequent recurrent cases: daily dose 1.5 mg / kg, children; daily dose 2.5 mg / kg. Cases showing resistance to steroids: 3 mg / kg daily, pediatric: 5 mg / kg daily
⑩ Daily dose of 5 mg/kg divided into twice daily. When effect was observed gradually decrease, keep: 3 mg / kg
⑪ Adult daily dose 3 mg / kg divided into twice daily, increasing or decreasing accordingly, not exceeding the daily dose of 5 mg / kg
Side effect
This drug has not conducted a survey that clarifies the frequency of occurrence of adverse reactions such as survey results.
1. Serious side effects (Frequency unknown)
1) Renal disorder: Renal function disorder is frequently observed as side effects of this drug, the main expression mechanism is thought to be due to dose-dependent renal vasoconstriction action, and usually recovered by weight loss or withdrawal [ BUN elevation, creatinine elevation showing renal blood flow reduction.
Usage notes
(Contraindications)
This drug ingredient hypersensitivity history history, pregnant women, potential for pregnancy, lactating women, tacrolimus (excluding topical preparations), pitavastatin, rosuvastatin, bosentan, aliskiren while taking colchicine in patients with liver / kidney disorder ) Neuro Behcet's Disease (Warning) This drug administration in organ transplantation should be performed under the guidance of a doctor who is familiar with immunosuppressive therapy and transplant patient management. In the case of atopic dermatitis, administration of this drug to a doctor who is familiar with the treatment of atopic dermatitis sufficiently explained the effectiveness and danger to the patient or its family in advance and confirmed that they understood Dosing should begin with. Since this drug is not biologically equivalent to Sandimmun (liquid for internal use or capsule) and its bioavailability is improved, when switching from Sandimmun to this drug, the concentration of cyclosporin in blood (AUC, Cmax ) To raise the side effects caused by the rise.
 环孢素A胶囊CICLOSPORIN Capsules 10mg[/caption]
药店国别:
产地国家:日本
处 方 药:是
所属类别:10毫克/胶囊 100胶囊/盒
包装规格:10毫克/胶囊 100胶囊/盒
计价单位:盒
生产厂家中文参考译名:富士药业
生产厂家英文名:Fuji Pharmaceutical
原产地英文商品名:CICLOSPORIN10mg/Capsules 100Capsules
原产地英文药品名:Ciclosporin
中文参考商品译名:CICLOSPORIN胶囊10毫克/胶囊 100胶囊/盒
中文参考药品译名:环孢素
简介:
部份中文環孢素A處方資料(僅供參考)
药物分类名称:免疫抑制剂
批准日期:2011年6月
欧文商标名:CICLOSPORIN Capsules
一般名:シクロスポリン (Ciclosporin)
別名:サイクロスポリンA
化学名:cyclo {-[(2S ,3R ,4R ,6E )- 3-Hydroxy-4-methyl-2-methylaminooct-6-enoyl]-L-2-aminobutanoyl-N -methylglycyl-N -methyl-L-leucyl-L-valyl-N-methyl-L-leucyl-L-alanyl-D-alanyl-N-methyl-L-leucyl-N-methyl-L-leucyl-N -methyl-L-valyl-}
分子式:C62H111N11O12
分子量:1202.61
性状:
它是一种白色的粉末。
它非常易溶于乙腈,甲醇或乙醇(95),易溶于乙醚,难溶于水。
处理注意事项:
“稳定性测试”
(1)环孢菌素胶囊10 mg“BMD”环孢菌素胶囊10 mg“BMD”在正常市场循环下使用最终包装产品“加速试验(40°C,相对湿度75%,6个月”)稳定3年,据推测
(2)环孢菌素胶囊25mg“BMD”作为使用最终包装产品的长期保存试验(室温保存3年)的结果,外观和含量等在规格范围内,环孢菌素胶囊25mg“BMD”经证实,在经销3年后稳定。
(3)环孢菌素胶囊50mg“BMD”使用最终包装产品,外观和含量的长期储存试验(室温保存3年)的结果在规格范围内,环孢素胶囊50mg“BMD”经证实,在经销3年后稳定。
由于胶囊可能由于吸湿而变软,因此请将它们保持在PTP包装中,直到服用。
适应病症:
在以下器官移植中抑制排斥反应
1.肾移植,肝移植,心脏移植,肺移植,胰移植,小肠移植
2.在骨髓移植中抑制排斥反应和移植物抗宿主病
3.白塞病(在眼部症状存在时)和其他非感染性葡萄膜炎(在现有治疗中有效,活跃部位具有降低非感染性葡萄膜炎视力风险)有限的)
4.寻常型银屑病(皮肤发炎超过全身的30%或难治性的情况),脓疱性牛皮癣,银屑病性红皮病,关节病牛皮癣。
5.再生障碍性贫血(严重),成红细胞。
6.肾病综合征(频繁复发或抵抗类固醇的病例)
7.系统型重症肌无力(在胸腺切除术后由于副作用引起的类固醇药物无效或难治)
8.特应性皮炎(无法获得现有治疗效果的患者)
用法与用量:
1.在肾移植的情况下
通常,作为移植前1天的环孢菌素,日剂量为9〜12mg/kg,每天口服两次,然后每天减少2mg / kg。 标准维持剂量为每日4〜6 mg / kg,但应根据症状进行增减。
2.在肝移植的情况下
通常,作为移植前1天的环孢菌素,每日口服两次给药,每日剂量为14〜16mg / kg。 此后,剂量逐渐降低,维持剂量设定为5〜10mg / kg /日标准,但根据症状适当增减。
3.在心脏移植的情况下,肺移植,胰腺移植
通常,从移植前1天起,每天口服两次,每天口服10〜15mg/kg,作为环孢菌素。 此后,剂量逐渐降低,维持剂量设定为2〜6mg /kg/日,作为标准,但根据症状适当增减。
4.在小肠移植的情况下
通常,环孢菌素以14至16mg/kg的日剂量口服给药,每天分成两次。 此后,剂量逐渐降低,维持剂量设定为5〜10mg / kg /日标准,但根据症状适当增减。 然而,从正常移植前1天开始注射环孢菌素后,可以口服给药,尽快切换口服。
5.在骨髓移植的情况下
通常,移植前1天,日剂量为6〜12mg/kg,每日口服2次,作为环孢素,持续3〜6个月,然后逐渐减量停止。
6.在贝切特病和其他非传染性葡萄膜炎的情况下
通常,口服给药是以每日两次5mg/kg的日剂量作为环孢菌素分开开始,然后每月每天减少或增加1〜2mg / kg。 维持剂量为每日3〜5mg / kg,作为标准,但应按适当的症状增加或减少。
7.在牛皮癣的情况下
通常,每日剂量为5mg/kg以两个分剂量施用。 如果观察到效果,每月减少1毫克/公斤/天,维持剂量为每日3毫克/公斤。 此外,应该根据症状增加或减少。
8.在再生障碍性贫血的情况下
通常,每天两次,以6mg/kg的日剂量口服给予环孢菌素。 此外,应该根据症状增加或减少。 此外,由于具有短的疾病持续时间的患者可能具有更好的治疗效果,因此期望将疾病持续时间少于6个月的患者作为指导。
9.肾病综合征:
通常,口服以下剂量作为环孢菌素分成一天两次。 此外,应该根据症状增加或减少。
(1) 频繁的复发病例
对于成年人,施用1.5mg/kg的日剂量。 在儿童的情况下,每天服用2.5mg/kg。
(2) 显示对类固醇抵抗的病例
对于成年人,每日给药3mg/kg。 在儿童的情况下,每天服用5毫克/千克。
10.在系统性重症肌无力的情况下
通常每天口服5毫克/千克作为环孢素分成两天。 如果观察到效果,逐渐降低剂量,维持3 mg/kg为标准。 此外,应该根据症状增加或减少。
11.在特应性皮炎的情况下
通常,成人每天口服3mg/kg,因为环孢菌素每天分成两次。 此外,应该根据症状增加或减少,但每天不应超过5毫克/千克
包装规格:
环孢菌素胶囊
10mg“BMD”100粒(10粒×10; PTP)
25 mg“BMD”100粒(10粒×10; PTP)
50 mg“BMD”100粒(10粒×10; PTP)
制造厂商:富士药业
Cyclosporine capsule 10 mg
Indication
① Suppression of rejection in kidney transplant
② Suppression of rejection in liver transplantation
③ Suppression of rejection in heart transplantation, lung transplantation and pancreatic transplantation
④ Suppression of rejection in small bowel transplantation
⑤ Suppression of rejection and graft-versus-host disease in bone marrow transplantation
⑥ Behcet's disease (in the presence of ocular symptoms) and other non-infectious uveitis (in non-infectious uveitis in the middle or the rear of the activity which is ineffective in existing treatments, Limited)
⑦ Vulgaris psoriasis (in cases where the skin eruption is over 30% of the whole body or if it is refractory), pustular psoriasis, psoriatic erythrosis, arthropathic psoriasis
⑧ aplastic anemia (severe), erythroblasts
⑨ Nephrotic syndrome (in cases of frequent recurrence or resistance to steroids)
⑩ Whole body type myasthenia gravis (in cases where administration of steroid drug is ineffective or difficult due to side effects in treatment after thymectomy)
⑪ Atopic dermatitis (a patient who can not obtain sufficient effect by the existing treatment
Usage · capacity
① From 1 day before transplantation, daily dose of 9 to 12 mg / kg is divided into two doses twice daily, and then the dose is reduced by 2 mg / kg daily. Maintenance amount: daily dose 4 to 6 mg / kg
② From 1 day before transplantation, 14-16 mg/kg per day is divided into two doses twice a day, then gradually reduced, maintenance dose: 5 to 10 mg / kg daily
③ From 1 day before the transplantation, 10 to 15 mg/kg per day is distributed twice a day, then gradually reduced, maintenance dose: 2 to 6 mg / kg daily
④ Daily dose of 14 to 16 mg/kg divided into twice daily. Starting with cyclosporine injection starting from 1 day before usual transplantation, administration is switched to oral administration as soon as possible after oral administration becomes possible
⑤ From 6 days before transplantation, 6 to 12 mg/kg is administered orally twice a day, continued for 3 to 6 months, then gradually decreased and discontinued
⑥ Start daily dose 5 mg/kg in divided doses twice a day, then reduce or increase 1 to 2 mg / kg per day every other month. Maintenance dose: daily dose 3 to 5 mg / kg
⑦ Daily dose 5 mg/kg divided into two doses. When effect is observed 1 mg / kg per day is reduced every 1 month, maintenance amount: 3 mg / kg per day
⑧ Daily dose of 6 mg/kg divided into twice daily. It is advisable to target patients with disease duration of less than 6 months
⑨ Apply the next dose to twice daily. Frequent recurrent cases: daily dose 1.5 mg / kg, children; daily dose 2.5 mg / kg. Cases showing resistance to steroids: 3 mg / kg daily, pediatric: 5 mg / kg daily
⑩ Daily dose of 5 mg/kg divided into twice daily. When effect was observed gradually decrease, keep: 3 mg / kg
⑪ Adult daily dose 3 mg / kg divided into twice daily, increasing or decreasing accordingly, not exceeding the daily dose of 5 mg / kg
Side effect
This drug has not conducted a survey that clarifies the frequency of occurrence of adverse reactions such as survey results.
1. Serious side effects (Frequency unknown)
1) Renal disorder: Renal function disorder is frequently observed as side effects of this drug, the main expression mechanism is thought to be due to dose-dependent renal vasoconstriction action, and usually recovered by weight loss or withdrawal [ BUN elevation, creatinine elevation showing renal blood flow reduction.
Usage notes
(Contraindications)
This drug ingredient hypersensitivity history history, pregnant women, potential for pregnancy, lactating women, tacrolimus (excluding topical preparations), pitavastatin, rosuvastatin, bosentan, aliskiren while taking colchicine in patients with liver / kidney disorder ) Neuro Behcet's Disease (Warning) This drug administration in organ transplantation should be performed under the guidance of a doctor who is familiar with immunosuppressive therapy and transplant patient management. In the case of atopic dermatitis, administration of this drug to a doctor who is familiar with the treatment of atopic dermatitis sufficiently explained the effectiveness and danger to the patient or its family in advance and confirmed that they understood Dosing should begin with. Since this drug is not biologically equivalent to Sandimmun (liquid for internal use or capsule) and its bioavailability is improved, when switching from Sandimmun to this drug, the concentration of cyclosporin in blood (AUC, Cmax ) To raise the side effects caused by the rise.
环孢素A胶囊CICLOSPORIN Capsules 10mg[/caption]
药店国别:
产地国家:日本
处 方 药:是
所属类别:10毫克/胶囊 100胶囊/盒
包装规格:10毫克/胶囊 100胶囊/盒
计价单位:盒
生产厂家中文参考译名:富士药业
生产厂家英文名:Fuji Pharmaceutical
原产地英文商品名:CICLOSPORIN10mg/Capsules 100Capsules
原产地英文药品名:Ciclosporin
中文参考商品译名:CICLOSPORIN胶囊10毫克/胶囊 100胶囊/盒
中文参考药品译名:环孢素
简介:
部份中文環孢素A處方資料(僅供參考)
药物分类名称:免疫抑制剂
批准日期:2011年6月
欧文商标名:CICLOSPORIN Capsules
一般名:シクロスポリン (Ciclosporin)
別名:サイクロスポリンA
化学名:cyclo {-[(2S ,3R ,4R ,6E )- 3-Hydroxy-4-methyl-2-methylaminooct-6-enoyl]-L-2-aminobutanoyl-N -methylglycyl-N -methyl-L-leucyl-L-valyl-N-methyl-L-leucyl-L-alanyl-D-alanyl-N-methyl-L-leucyl-N-methyl-L-leucyl-N -methyl-L-valyl-}
分子式:C62H111N11O12
分子量:1202.61
性状:
它是一种白色的粉末。
它非常易溶于乙腈,甲醇或乙醇(95),易溶于乙醚,难溶于水。
处理注意事项:
“稳定性测试”
(1)环孢菌素胶囊10 mg“BMD”环孢菌素胶囊10 mg“BMD”在正常市场循环下使用最终包装产品“加速试验(40°C,相对湿度75%,6个月”)稳定3年,据推测
(2)环孢菌素胶囊25mg“BMD”作为使用最终包装产品的长期保存试验(室温保存3年)的结果,外观和含量等在规格范围内,环孢菌素胶囊25mg“BMD”经证实,在经销3年后稳定。
(3)环孢菌素胶囊50mg“BMD”使用最终包装产品,外观和含量的长期储存试验(室温保存3年)的结果在规格范围内,环孢素胶囊50mg“BMD”经证实,在经销3年后稳定。
由于胶囊可能由于吸湿而变软,因此请将它们保持在PTP包装中,直到服用。
适应病症:
在以下器官移植中抑制排斥反应
1.肾移植,肝移植,心脏移植,肺移植,胰移植,小肠移植
2.在骨髓移植中抑制排斥反应和移植物抗宿主病
3.白塞病(在眼部症状存在时)和其他非感染性葡萄膜炎(在现有治疗中有效,活跃部位具有降低非感染性葡萄膜炎视力风险)有限的)
4.寻常型银屑病(皮肤发炎超过全身的30%或难治性的情况),脓疱性牛皮癣,银屑病性红皮病,关节病牛皮癣。
5.再生障碍性贫血(严重),成红细胞。
6.肾病综合征(频繁复发或抵抗类固醇的病例)
7.系统型重症肌无力(在胸腺切除术后由于副作用引起的类固醇药物无效或难治)
8.特应性皮炎(无法获得现有治疗效果的患者)
用法与用量:
1.在肾移植的情况下
通常,作为移植前1天的环孢菌素,日剂量为9〜12mg/kg,每天口服两次,然后每天减少2mg / kg。 标准维持剂量为每日4〜6 mg / kg,但应根据症状进行增减。
2.在肝移植的情况下
通常,作为移植前1天的环孢菌素,每日口服两次给药,每日剂量为14〜16mg / kg。 此后,剂量逐渐降低,维持剂量设定为5〜10mg / kg /日标准,但根据症状适当增减。
3.在心脏移植的情况下,肺移植,胰腺移植
通常,从移植前1天起,每天口服两次,每天口服10〜15mg/kg,作为环孢菌素。 此后,剂量逐渐降低,维持剂量设定为2〜6mg /kg/日,作为标准,但根据症状适当增减。
4.在小肠移植的情况下
通常,环孢菌素以14至16mg/kg的日剂量口服给药,每天分成两次。 此后,剂量逐渐降低,维持剂量设定为5〜10mg / kg /日标准,但根据症状适当增减。 然而,从正常移植前1天开始注射环孢菌素后,可以口服给药,尽快切换口服。
5.在骨髓移植的情况下
通常,移植前1天,日剂量为6〜12mg/kg,每日口服2次,作为环孢素,持续3〜6个月,然后逐渐减量停止。
6.在贝切特病和其他非传染性葡萄膜炎的情况下
通常,口服给药是以每日两次5mg/kg的日剂量作为环孢菌素分开开始,然后每月每天减少或增加1〜2mg / kg。 维持剂量为每日3〜5mg / kg,作为标准,但应按适当的症状增加或减少。
7.在牛皮癣的情况下
通常,每日剂量为5mg/kg以两个分剂量施用。 如果观察到效果,每月减少1毫克/公斤/天,维持剂量为每日3毫克/公斤。 此外,应该根据症状增加或减少。
8.在再生障碍性贫血的情况下
通常,每天两次,以6mg/kg的日剂量口服给予环孢菌素。 此外,应该根据症状增加或减少。 此外,由于具有短的疾病持续时间的患者可能具有更好的治疗效果,因此期望将疾病持续时间少于6个月的患者作为指导。
9.肾病综合征:
通常,口服以下剂量作为环孢菌素分成一天两次。 此外,应该根据症状增加或减少。
(1) 频繁的复发病例
对于成年人,施用1.5mg/kg的日剂量。 在儿童的情况下,每天服用2.5mg/kg。
(2) 显示对类固醇抵抗的病例
对于成年人,每日给药3mg/kg。 在儿童的情况下,每天服用5毫克/千克。
10.在系统性重症肌无力的情况下
通常每天口服5毫克/千克作为环孢素分成两天。 如果观察到效果,逐渐降低剂量,维持3 mg/kg为标准。 此外,应该根据症状增加或减少。
11.在特应性皮炎的情况下
通常,成人每天口服3mg/kg,因为环孢菌素每天分成两次。 此外,应该根据症状增加或减少,但每天不应超过5毫克/千克
包装规格:
环孢菌素胶囊
10mg“BMD”100粒(10粒×10; PTP)
25 mg“BMD”100粒(10粒×10; PTP)
50 mg“BMD”100粒(10粒×10; PTP)
制造厂商:富士药业
Cyclosporine capsule 10 mg
Indication
① Suppression of rejection in kidney transplant
② Suppression of rejection in liver transplantation
③ Suppression of rejection in heart transplantation, lung transplantation and pancreatic transplantation
④ Suppression of rejection in small bowel transplantation
⑤ Suppression of rejection and graft-versus-host disease in bone marrow transplantation
⑥ Behcet's disease (in the presence of ocular symptoms) and other non-infectious uveitis (in non-infectious uveitis in the middle or the rear of the activity which is ineffective in existing treatments, Limited)
⑦ Vulgaris psoriasis (in cases where the skin eruption is over 30% of the whole body or if it is refractory), pustular psoriasis, psoriatic erythrosis, arthropathic psoriasis
⑧ aplastic anemia (severe), erythroblasts
⑨ Nephrotic syndrome (in cases of frequent recurrence or resistance to steroids)
⑩ Whole body type myasthenia gravis (in cases where administration of steroid drug is ineffective or difficult due to side effects in treatment after thymectomy)
⑪ Atopic dermatitis (a patient who can not obtain sufficient effect by the existing treatment
Usage · capacity
① From 1 day before transplantation, daily dose of 9 to 12 mg / kg is divided into two doses twice daily, and then the dose is reduced by 2 mg / kg daily. Maintenance amount: daily dose 4 to 6 mg / kg
② From 1 day before transplantation, 14-16 mg/kg per day is divided into two doses twice a day, then gradually reduced, maintenance dose: 5 to 10 mg / kg daily
③ From 1 day before the transplantation, 10 to 15 mg/kg per day is distributed twice a day, then gradually reduced, maintenance dose: 2 to 6 mg / kg daily
④ Daily dose of 14 to 16 mg/kg divided into twice daily. Starting with cyclosporine injection starting from 1 day before usual transplantation, administration is switched to oral administration as soon as possible after oral administration becomes possible
⑤ From 6 days before transplantation, 6 to 12 mg/kg is administered orally twice a day, continued for 3 to 6 months, then gradually decreased and discontinued
⑥ Start daily dose 5 mg/kg in divided doses twice a day, then reduce or increase 1 to 2 mg / kg per day every other month. Maintenance dose: daily dose 3 to 5 mg / kg
⑦ Daily dose 5 mg/kg divided into two doses. When effect is observed 1 mg / kg per day is reduced every 1 month, maintenance amount: 3 mg / kg per day
⑧ Daily dose of 6 mg/kg divided into twice daily. It is advisable to target patients with disease duration of less than 6 months
⑨ Apply the next dose to twice daily. Frequent recurrent cases: daily dose 1.5 mg / kg, children; daily dose 2.5 mg / kg. Cases showing resistance to steroids: 3 mg / kg daily, pediatric: 5 mg / kg daily
⑩ Daily dose of 5 mg/kg divided into twice daily. When effect was observed gradually decrease, keep: 3 mg / kg
⑪ Adult daily dose 3 mg / kg divided into twice daily, increasing or decreasing accordingly, not exceeding the daily dose of 5 mg / kg
Side effect
This drug has not conducted a survey that clarifies the frequency of occurrence of adverse reactions such as survey results.
1. Serious side effects (Frequency unknown)
1) Renal disorder: Renal function disorder is frequently observed as side effects of this drug, the main expression mechanism is thought to be due to dose-dependent renal vasoconstriction action, and usually recovered by weight loss or withdrawal [ BUN elevation, creatinine elevation showing renal blood flow reduction.
Usage notes
(Contraindications)
This drug ingredient hypersensitivity history history, pregnant women, potential for pregnancy, lactating women, tacrolimus (excluding topical preparations), pitavastatin, rosuvastatin, bosentan, aliskiren while taking colchicine in patients with liver / kidney disorder ) Neuro Behcet's Disease (Warning) This drug administration in organ transplantation should be performed under the guidance of a doctor who is familiar with immunosuppressive therapy and transplant patient management. In the case of atopic dermatitis, administration of this drug to a doctor who is familiar with the treatment of atopic dermatitis sufficiently explained the effectiveness and danger to the patient or its family in advance and confirmed that they understood Dosing should begin with. Since this drug is not biologically equivalent to Sandimmun (liquid for internal use or capsule) and its bioavailability is improved, when switching from Sandimmun to this drug, the concentration of cyclosporin in blood (AUC, Cmax ) To raise the side effects caused by the rise.
用药温馨提示:当您服用此药物时,需定期接受医疗专业人士的检查,以便随时针对其药效、副作用等情况进行监测。本网站所包含的信息旨在为患者提供帮助,不能代替医学建议和治疗。
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 环孢素A胶囊CICLOSPORIN Capsules 10mg说明书
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 环孢素A胶囊CICLOSPORIN Capsules 10mg说明书




![免疫球蛋白输液[人类]HyQvia Infusionslösung 100mg/ml 5g说明书](https://www.yschn.com/wp-content/uploads/2020/07/1594708651-ea416acbfaad633.gif)
