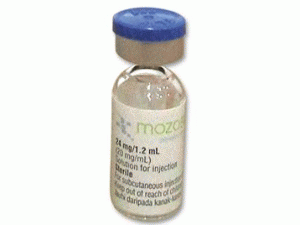
普乐沙福注射液MOZOBIL Inj 24mg/1.2ml(plerixafor)
 药店国别:
产地国家:瑞士
处方药:是
所属类别: 24毫克/毫升 1.2毫升/瓶
包装规格: 24毫克/毫升 1.2毫升/瓶
计价单位:瓶
生产厂家中文参考译名:
生产厂家英文名:Sanofi-Aventis (Suisse) SA
原产地英文商品名:MOZOBIL Inj 24 mg/1.2ml/vial
原产地英文药品名:PLERIXAFOR
中文参考商品译名:MOZOBIL注射溶液 24毫克/毫升 1.2毫升/瓶
中文参考药品译名:普乐沙福
曾用名:
简介:部份中文普乐沙福注射液处方资料(仅供参考)通用名称:普乐沙福注射液英文名称:plerixafor injection生产厂家:赛诺菲 - 安万特药品简介2009年8月6日,抗癌症新药Mozobil获得欧盟批准上市,对淋巴瘤与多发性骨髓瘤患者提供重要的选项,这些患者原本需要异体干细胞移植作用。
剂型:
注射液规格:24mg/1.2ml
作用机理
Plerixafor是CXCR4的趋化因子受体并阻断其同源配体,基质细胞衍生因子1α(SDF-1α)的结合的抑制剂。 SDF-1α和CXCR4被认为发挥贩运和人造血干细胞(HSC)的归巢作用是骨髓舱。一旦在骨髓,干细胞的CXCR4可起到帮助锚定这些细胞的骨髓基质,可以通过SDF-1α或通过其他粘附分子的诱导直接。与plerixafor治疗导致白细胞增多和海拔在小鼠,狗和人造血循环祖细胞。通过动员plerixafor CD34 +细胞能植入与长期再生能力长达一年犬移植模型。
适应症和用法
Mozobil,造血干细胞动员,指示结合粒细胞集落刺激因子(G-CSF),以动员造血干细胞的外周血收集和随后的自体移植的患者的非霍奇金淋巴瘤和多发性骨髓瘤。
用法用量
•启动治疗Mozobil患者接受的G-CSF后,每天一次,4天。•重复Mozobil剂量最多连续4天。•选择剂量以0.24毫克/公斤实际体重。•通过单采开始大约11小时前,皮下注射辖。•肾损害:如果肌酐清除率≤50mL/min的剂量减少三分之一至0.16毫克/公斤。剂型和规格•含有20毫克/毫升溶液1.2毫升一次性使用小瓶中。
禁忌
•无。
普乐沙福注射液英文版说明书
Mozobil (Plerixafor Injection)Raise your expectations for apheresis success in non-Hodgkin's lymphoma (NHL) and multiple myleoma (MM) patients with Mozobil+granulocyte-colony stimulating factor (G-CSF) compared to G-CSF alone.IndicationMozobil (plerixafor injection) is indicated in combination with granulocyte-colony stimulating factor (G-CSF) to mobilize hematopoietic stem cells (HSCs) to the peripheral blood for collection and subsequent autologous transplantation in patients with non-Hodgkin's lymphoma (NHL) and multiple myeloma (MM).Important Safety Information for Mozobil (plerixafor injection)•Severe, life-threatening allergic reactions (anaphylaxis) can happen in people who take Mozobil. Tell your doctor right away if you experience hives (itchy raised bumps), eye swelling, or trouble breathing.•Mozobil is not intended for hematopoietic stem cell transplantation (HSCT) mobilization and collection in patients with leukemia.•Mozobil in combination with G-CSF increases circulating white blood cells (WBCs). Your WBC counts will be monitored.•Thrombocytopenia (a decrease in the number of platelets circulating in the blood) has been observed in patients receiving Mozobil. Your platelet counts will be monitored.•Cancer cells may be released from the bone marrow and subsequently collected along with your stem cells during apheresis. The potential effects of infusing cancer cells during your transplant have not been well-studied.•Your spleen may be examined if you experience pain in the left upper stomach area or left shoulder area as these may be signs of an enlarged or burst (ruptured) spleen.•Mozobil may harm the unborn child when administered to a pregnant woman. Scientific studies have shown that Mozobil causes harm to unborn animals. The safety of Mozobil in pregnant women has not been established in clinical trials.If you are of childbearing potential you should be advised to avoid becoming pregnant while receiving treatment with Mozobil. If this drug is used during pregnancy, or if you become pregnant while taking this drug, you should be apprised of the potential hazard to the unborn child.•The most common adverse reactions (occurring in greater than or equal to 10% of patients) during HSC mobilization and apheresis were: diarrhea (37%), nausea (34%), tiredness (fatigue) (27%), injection site reactions (34%), headache (22%), pain in your joints (arthralgia) (13%), dizziness (11%), and vomiting (10%).
药店国别:
产地国家:瑞士
处方药:是
所属类别: 24毫克/毫升 1.2毫升/瓶
包装规格: 24毫克/毫升 1.2毫升/瓶
计价单位:瓶
生产厂家中文参考译名:
生产厂家英文名:Sanofi-Aventis (Suisse) SA
原产地英文商品名:MOZOBIL Inj 24 mg/1.2ml/vial
原产地英文药品名:PLERIXAFOR
中文参考商品译名:MOZOBIL注射溶液 24毫克/毫升 1.2毫升/瓶
中文参考药品译名:普乐沙福
曾用名:
简介:部份中文普乐沙福注射液处方资料(仅供参考)通用名称:普乐沙福注射液英文名称:plerixafor injection生产厂家:赛诺菲 - 安万特药品简介2009年8月6日,抗癌症新药Mozobil获得欧盟批准上市,对淋巴瘤与多发性骨髓瘤患者提供重要的选项,这些患者原本需要异体干细胞移植作用。
剂型:
注射液规格:24mg/1.2ml
作用机理
Plerixafor是CXCR4的趋化因子受体并阻断其同源配体,基质细胞衍生因子1α(SDF-1α)的结合的抑制剂。 SDF-1α和CXCR4被认为发挥贩运和人造血干细胞(HSC)的归巢作用是骨髓舱。一旦在骨髓,干细胞的CXCR4可起到帮助锚定这些细胞的骨髓基质,可以通过SDF-1α或通过其他粘附分子的诱导直接。与plerixafor治疗导致白细胞增多和海拔在小鼠,狗和人造血循环祖细胞。通过动员plerixafor CD34 +细胞能植入与长期再生能力长达一年犬移植模型。
适应症和用法
Mozobil,造血干细胞动员,指示结合粒细胞集落刺激因子(G-CSF),以动员造血干细胞的外周血收集和随后的自体移植的患者的非霍奇金淋巴瘤和多发性骨髓瘤。
用法用量
•启动治疗Mozobil患者接受的G-CSF后,每天一次,4天。•重复Mozobil剂量最多连续4天。•选择剂量以0.24毫克/公斤实际体重。•通过单采开始大约11小时前,皮下注射辖。•肾损害:如果肌酐清除率≤50mL/min的剂量减少三分之一至0.16毫克/公斤。剂型和规格•含有20毫克/毫升溶液1.2毫升一次性使用小瓶中。
禁忌
•无。
普乐沙福注射液英文版说明书
Mozobil (Plerixafor Injection)Raise your expectations for apheresis success in non-Hodgkin's lymphoma (NHL) and multiple myleoma (MM) patients with Mozobil+granulocyte-colony stimulating factor (G-CSF) compared to G-CSF alone.IndicationMozobil (plerixafor injection) is indicated in combination with granulocyte-colony stimulating factor (G-CSF) to mobilize hematopoietic stem cells (HSCs) to the peripheral blood for collection and subsequent autologous transplantation in patients with non-Hodgkin's lymphoma (NHL) and multiple myeloma (MM).Important Safety Information for Mozobil (plerixafor injection)•Severe, life-threatening allergic reactions (anaphylaxis) can happen in people who take Mozobil. Tell your doctor right away if you experience hives (itchy raised bumps), eye swelling, or trouble breathing.•Mozobil is not intended for hematopoietic stem cell transplantation (HSCT) mobilization and collection in patients with leukemia.•Mozobil in combination with G-CSF increases circulating white blood cells (WBCs). Your WBC counts will be monitored.•Thrombocytopenia (a decrease in the number of platelets circulating in the blood) has been observed in patients receiving Mozobil. Your platelet counts will be monitored.•Cancer cells may be released from the bone marrow and subsequently collected along with your stem cells during apheresis. The potential effects of infusing cancer cells during your transplant have not been well-studied.•Your spleen may be examined if you experience pain in the left upper stomach area or left shoulder area as these may be signs of an enlarged or burst (ruptured) spleen.•Mozobil may harm the unborn child when administered to a pregnant woman. Scientific studies have shown that Mozobil causes harm to unborn animals. The safety of Mozobil in pregnant women has not been established in clinical trials.If you are of childbearing potential you should be advised to avoid becoming pregnant while receiving treatment with Mozobil. If this drug is used during pregnancy, or if you become pregnant while taking this drug, you should be apprised of the potential hazard to the unborn child.•The most common adverse reactions (occurring in greater than or equal to 10% of patients) during HSC mobilization and apheresis were: diarrhea (37%), nausea (34%), tiredness (fatigue) (27%), injection site reactions (34%), headache (22%), pain in your joints (arthralgia) (13%), dizziness (11%), and vomiting (10%).
用药温馨提示:当您服用此药物时,需定期接受医疗专业人士的检查,以便随时针对其药效、副作用等情况进行监测。本网站所包含的信息旨在为患者提供帮助,不能代替医学建议和治疗。
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 普乐沙福注射液MOZOBIL Inj 24mg/1.2ml(plerixafor)
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 普乐沙福注射液MOZOBIL Inj 24mg/1.2ml(plerixafor)