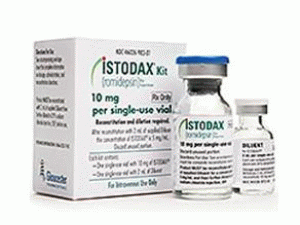
罗米地辛冻干粉注射剂ISTODAX KIT FOR INJ 10MG
 药店国别:
产地国家:美国
处方药:是
所属类别: 10毫克/盒
包装规格: 10毫克/盒
计价单位:盒
生产厂家中文参考译名:
生产厂家英文名:Gloucester Pharmaceutical
原产地英文商品名:ISTODAX KIT FOR INJ 10MG SDV DPSH 1/EA
原产地英文药品名:ROMIDEPSIN
中文参考商品译名:ISTODAX套件 10毫克/盒
中文参考药品译名:罗米地辛
曾用名:
简介:近日,由美国GloucesterPharmaceuticals公司开发的抗癌药物romidepsin(商品名为Istodax)于2009年11月9日获得美国FDA批准上市,该药用于治疗皮肤T细胞淋巴瘤(CTCL)。批准日期:2009年11月5日;公司:GloucesterPharmaceuticalsSTODAX KIT(罗米地辛[romidepsin]) 注射液,供静脉注射使用美国最初批准:2009
作用机制
Romidepsin是一种组蛋白去乙酰化酶(HDAC)抑制剂。HDACs在组蛋白中催化从乙酰化赖氨酸残基去除乙酰基,导致基因表达的调节。HDACs还脱去乙酰基非-组蛋白蛋白,例如转录因子。在体外,romidepsin引起乙酰化组蛋白的蓄积,和诱导细胞周期停止和某些癌症细胞株凋亡有IC50值在纳克分子范围。在非临床和临床研究中观察到Romidepsin的抗肿瘤作用机制的特点尚未完全确定。
适应证和用途
ISTODAX是一种组蛋白去乙酰化酶(HDAC)抑制剂适用于:(1)在已接受至少1次既往全身治疗的患者中皮肤T-细胞淋巴瘤的治疗(CTCL).
剂量和给药方法
(1)28天疗程的第1,8和15天分别在4小时过程静脉输注14mg/m2。每28天重复1个疗程,提供患者继续获益并耐受药物。
(2)为处理不良药物反应可能需要停止治疗或中断本品,减低或不减低剂量至10mg/m2。剂型和规格注射用ISTODAX,10mg,提供1小瓶含2mL(可输送容积)稀释液。
禁忌证
无。
警告和注意事项
(1)由于QT延长的风险给予ISTODAX前确保钾和镁是在正常范围内。
(2)用ISTODAX治疗曾伴有血小板减少,白细胞减少(中性粒细胞减少和淋巴细胞减少),和贫血;所以,用ISTODAX治疗期间监查这些血液学参数,必要时修改剂量。
(3)曾观察到心电图(ECG)变化。有先天性长QT综合征患者中,明显心血管病史,和使用导致显著QT延长药品患者,考虑心血管监查警惕。
(4)根据其作用机制,ISTODAX可能引起胎儿危害当给予妊娠妇女。劝告妇女对胎儿潜在危害。
(5)ISTODAX与雌激素受体结合。劝告有生育潜能妇女ISTODAX可能 may 减低含雌激素避孕药的疗效。
不良反应
在研究1中最常见不良反应是恶心,疲劳,感染,呕吐,和厌食;而在研究2中为恶心,疲劳,贫血,血小板减少,ECGT-波变化,中性粒细胞减少,和淋巴细胞减少。
药物相互作用
(1)在同时给予ISTODAX和香豆定[Coumadin]衍生物患者中仔细监查凝血酶原时间(PT)和国际标准化比率(INR)。
(2)强CYP3A4抑制剂可能增加ISTODAX的浓度和应当避免。
(3)强CYP3A4诱导剂可能减低ISTODAX的浓度和应当避免
罗米地辛冻干粉注射剂英文版说明书
ISTODAX® is indicated for treatment of cutaneous T-celllymphoma (CTCL) in patients who have received atleast one prior systemic therapy.ISTODAX® is indicated for treatment of peripheral T-celllymphoma (PTCL) in patients who have received atleast one prior therapy.These indications are based on response rate. Clinicalbenefit such as improvement in overall survival has notbeen demonstrated.Important Safety InformationWARNINGS AND PRECAUTIONS•Treatment with ISTODAX® (romidepsin) has been associated with thrombocytopenia, leukopenia (neutropenia and lymphopenia), and anemia; therefore, monitor these hematological parameters during treatment with ISTODAX and modify the dose as necessary•Serious and sometimes fatal infections have been reported during treatment and within 30 days after treatment with ISTODAX. The risk of life threatening infections may be higher in patients with a history of extensive or intensive chemotherapy•Electrocardiographic (ECG) changes have been observed with ISTODAX•In patients with congenital long QT syndrome, patients with a history of significant cardiovascular disease, and patients taking anti-arrhythmic medicines or medicinal products that lead to significant QT prolongation, appropriate cardiovascular monitoring precautions should be considered, such as monitoring electrolytes and ECGs at baseline and periodically during treatment•Ensure that potassium and magnesium are within the normal range before administration of ISTODAX•Tumor lysis syndrome has been reported during treatment with ISTODAX. Patients with advanced stage disease and/or high tumor burden should be closely monitored and appropriate precautions taken, and treatment should be instituted as appropriate•ISTODAX may cause fetal harm when administered to a pregnant woman. Advise women to avoid pregnancy while receiving ISTODAX. If this drug is used during pregnancy, or if the patient becomes pregnant while taking ISTODAX, the patient should be apprised of the potential hazard to the fetus (Pregnancy Category D)ADVERSE REACTIONSPeripheral T-Cell LymphomaThe most common Grade 3/4 adverse reactions (>5%) regardless of causality in Study 3 (N=131) were thrombocytopenia (24%), neutropenia (20%), anemia (11%), asthenia/fatigue (8%), and leukopenia (6%), and in Study 4 (N=47) were neutropenia (47%), leukopenia (45%), thrombocytopenia (36%), anemia (28%), asthenia/fatigue (19%), pyrexia (17%), vomiting (9%), and nausea (6%).Infections were the most common type of serious adverse event reported in Study 3 (N=131) and Study 4 (N=47). In Study 3, 25 patients (19%) experienced a serious infection, including 6 patients (5%) with serious treatment-related infections. In Study 4, 11 patients (23%) experienced a serious infection, including 8 patients (17%) with serious treatment-related infections.The most common adverse reactions regardless of causality in Study 3 (N=131) were nausea (59%), asthenia/fatigue (55%), thrombocytopenia (41%), vomiting (39%), diarrhea (36%), and pyrexia (35%), and in Study 4 (N=47) were asthenia/fatigue (77%), nausea (75%), thrombocytopenia (72%), neutropenia (66%), anemia (62%), leukopenia (55%), pyrexia (47%), anorexia (45%), vomiting (40%), constipation (40%), and diarrhea (36%).Cutaneous T-Cell LymphomaThe most common Grade 3/4 adverse reactions (>5%) regardless of causality in Study 1 (N=102) were infections (11%) and asthenia/fatigue (8%), and in Study 2 (N=83) were lymphopenia (37%), infections (33%), neutropenia (27%), leukopenia (22%), anemia (16%), asthenia/fatigue (14%), thrombocytopenia (14%), hypophosphatemia (10%), vomiting (10%), dermatitis/exfoliative dermatitis (8%), hypermagnesemia (8%), hyperuricemia (8%), hypocalcemia (6%), nausea (6%), and pruritus (6%).Infections were the most common type of serious adverse event reported in both Study 1 (N=102) and Study 2 (N=83) with 8 patients (8%) in Study 1 and 26 patients (31%) in Study 2 experiencing a serious infection.The most common adverse reactions regardless of causality in Study 1 (N=102) were nausea (56%), asthenia/fatigue (53%), infections (46%), vomiting (34%), and anorexia (23%), and in Study 2 (N=83) were nausea (86%), asthenia/fatigue (77%), anemia (72%), thrombocytopenia (65%), ECG ST-T wave changes (63%), neutropenia (57%), lymphopenia (57%), infections (54%), anorexia (54%), vomiting (52%), hypocalcemia (52%), hyperglycemia (51%), hypoalbuminemia (48%), leukopenia (46%), dysgeusia (40%), and constipation (39%).DRUG INTERACTIONS•Monitor prothrombin time and International Normalized Ratio in patients concurrently administered ISTODAX (romidepsin) and warfarin sodium derivatives•Romidepsin is metabolized by CYP3A4◦Monitor patients for toxicity related to increased romidepsin exposure and follow dose modifications for toxicity when ISTODAX is initially co-administered with strong CYP3A4 inhibitors◦Avoid co-administration of ISTODAX (romidepsin) with rifampin and other potent inducers of CYP3A4•Exercise caution with concomitant use of ISTODAX and P-glycoprotein (P-gp, ABCB1) inhibitorsUSE IN SPECIFIC POPULATIONS•Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from ISTODAX, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother•Patients with moderate and severe hepatic impairment and/or patients with end-stage renal disease should be treated with caution
药店国别:
产地国家:美国
处方药:是
所属类别: 10毫克/盒
包装规格: 10毫克/盒
计价单位:盒
生产厂家中文参考译名:
生产厂家英文名:Gloucester Pharmaceutical
原产地英文商品名:ISTODAX KIT FOR INJ 10MG SDV DPSH 1/EA
原产地英文药品名:ROMIDEPSIN
中文参考商品译名:ISTODAX套件 10毫克/盒
中文参考药品译名:罗米地辛
曾用名:
简介:近日,由美国GloucesterPharmaceuticals公司开发的抗癌药物romidepsin(商品名为Istodax)于2009年11月9日获得美国FDA批准上市,该药用于治疗皮肤T细胞淋巴瘤(CTCL)。批准日期:2009年11月5日;公司:GloucesterPharmaceuticalsSTODAX KIT(罗米地辛[romidepsin]) 注射液,供静脉注射使用美国最初批准:2009
作用机制
Romidepsin是一种组蛋白去乙酰化酶(HDAC)抑制剂。HDACs在组蛋白中催化从乙酰化赖氨酸残基去除乙酰基,导致基因表达的调节。HDACs还脱去乙酰基非-组蛋白蛋白,例如转录因子。在体外,romidepsin引起乙酰化组蛋白的蓄积,和诱导细胞周期停止和某些癌症细胞株凋亡有IC50值在纳克分子范围。在非临床和临床研究中观察到Romidepsin的抗肿瘤作用机制的特点尚未完全确定。
适应证和用途
ISTODAX是一种组蛋白去乙酰化酶(HDAC)抑制剂适用于:(1)在已接受至少1次既往全身治疗的患者中皮肤T-细胞淋巴瘤的治疗(CTCL).
剂量和给药方法
(1)28天疗程的第1,8和15天分别在4小时过程静脉输注14mg/m2。每28天重复1个疗程,提供患者继续获益并耐受药物。
(2)为处理不良药物反应可能需要停止治疗或中断本品,减低或不减低剂量至10mg/m2。剂型和规格注射用ISTODAX,10mg,提供1小瓶含2mL(可输送容积)稀释液。
禁忌证
无。
警告和注意事项
(1)由于QT延长的风险给予ISTODAX前确保钾和镁是在正常范围内。
(2)用ISTODAX治疗曾伴有血小板减少,白细胞减少(中性粒细胞减少和淋巴细胞减少),和贫血;所以,用ISTODAX治疗期间监查这些血液学参数,必要时修改剂量。
(3)曾观察到心电图(ECG)变化。有先天性长QT综合征患者中,明显心血管病史,和使用导致显著QT延长药品患者,考虑心血管监查警惕。
(4)根据其作用机制,ISTODAX可能引起胎儿危害当给予妊娠妇女。劝告妇女对胎儿潜在危害。
(5)ISTODAX与雌激素受体结合。劝告有生育潜能妇女ISTODAX可能 may 减低含雌激素避孕药的疗效。
不良反应
在研究1中最常见不良反应是恶心,疲劳,感染,呕吐,和厌食;而在研究2中为恶心,疲劳,贫血,血小板减少,ECGT-波变化,中性粒细胞减少,和淋巴细胞减少。
药物相互作用
(1)在同时给予ISTODAX和香豆定[Coumadin]衍生物患者中仔细监查凝血酶原时间(PT)和国际标准化比率(INR)。
(2)强CYP3A4抑制剂可能增加ISTODAX的浓度和应当避免。
(3)强CYP3A4诱导剂可能减低ISTODAX的浓度和应当避免
罗米地辛冻干粉注射剂英文版说明书
ISTODAX® is indicated for treatment of cutaneous T-celllymphoma (CTCL) in patients who have received atleast one prior systemic therapy.ISTODAX® is indicated for treatment of peripheral T-celllymphoma (PTCL) in patients who have received atleast one prior therapy.These indications are based on response rate. Clinicalbenefit such as improvement in overall survival has notbeen demonstrated.Important Safety InformationWARNINGS AND PRECAUTIONS•Treatment with ISTODAX® (romidepsin) has been associated with thrombocytopenia, leukopenia (neutropenia and lymphopenia), and anemia; therefore, monitor these hematological parameters during treatment with ISTODAX and modify the dose as necessary•Serious and sometimes fatal infections have been reported during treatment and within 30 days after treatment with ISTODAX. The risk of life threatening infections may be higher in patients with a history of extensive or intensive chemotherapy•Electrocardiographic (ECG) changes have been observed with ISTODAX•In patients with congenital long QT syndrome, patients with a history of significant cardiovascular disease, and patients taking anti-arrhythmic medicines or medicinal products that lead to significant QT prolongation, appropriate cardiovascular monitoring precautions should be considered, such as monitoring electrolytes and ECGs at baseline and periodically during treatment•Ensure that potassium and magnesium are within the normal range before administration of ISTODAX•Tumor lysis syndrome has been reported during treatment with ISTODAX. Patients with advanced stage disease and/or high tumor burden should be closely monitored and appropriate precautions taken, and treatment should be instituted as appropriate•ISTODAX may cause fetal harm when administered to a pregnant woman. Advise women to avoid pregnancy while receiving ISTODAX. If this drug is used during pregnancy, or if the patient becomes pregnant while taking ISTODAX, the patient should be apprised of the potential hazard to the fetus (Pregnancy Category D)ADVERSE REACTIONSPeripheral T-Cell LymphomaThe most common Grade 3/4 adverse reactions (>5%) regardless of causality in Study 3 (N=131) were thrombocytopenia (24%), neutropenia (20%), anemia (11%), asthenia/fatigue (8%), and leukopenia (6%), and in Study 4 (N=47) were neutropenia (47%), leukopenia (45%), thrombocytopenia (36%), anemia (28%), asthenia/fatigue (19%), pyrexia (17%), vomiting (9%), and nausea (6%).Infections were the most common type of serious adverse event reported in Study 3 (N=131) and Study 4 (N=47). In Study 3, 25 patients (19%) experienced a serious infection, including 6 patients (5%) with serious treatment-related infections. In Study 4, 11 patients (23%) experienced a serious infection, including 8 patients (17%) with serious treatment-related infections.The most common adverse reactions regardless of causality in Study 3 (N=131) were nausea (59%), asthenia/fatigue (55%), thrombocytopenia (41%), vomiting (39%), diarrhea (36%), and pyrexia (35%), and in Study 4 (N=47) were asthenia/fatigue (77%), nausea (75%), thrombocytopenia (72%), neutropenia (66%), anemia (62%), leukopenia (55%), pyrexia (47%), anorexia (45%), vomiting (40%), constipation (40%), and diarrhea (36%).Cutaneous T-Cell LymphomaThe most common Grade 3/4 adverse reactions (>5%) regardless of causality in Study 1 (N=102) were infections (11%) and asthenia/fatigue (8%), and in Study 2 (N=83) were lymphopenia (37%), infections (33%), neutropenia (27%), leukopenia (22%), anemia (16%), asthenia/fatigue (14%), thrombocytopenia (14%), hypophosphatemia (10%), vomiting (10%), dermatitis/exfoliative dermatitis (8%), hypermagnesemia (8%), hyperuricemia (8%), hypocalcemia (6%), nausea (6%), and pruritus (6%).Infections were the most common type of serious adverse event reported in both Study 1 (N=102) and Study 2 (N=83) with 8 patients (8%) in Study 1 and 26 patients (31%) in Study 2 experiencing a serious infection.The most common adverse reactions regardless of causality in Study 1 (N=102) were nausea (56%), asthenia/fatigue (53%), infections (46%), vomiting (34%), and anorexia (23%), and in Study 2 (N=83) were nausea (86%), asthenia/fatigue (77%), anemia (72%), thrombocytopenia (65%), ECG ST-T wave changes (63%), neutropenia (57%), lymphopenia (57%), infections (54%), anorexia (54%), vomiting (52%), hypocalcemia (52%), hyperglycemia (51%), hypoalbuminemia (48%), leukopenia (46%), dysgeusia (40%), and constipation (39%).DRUG INTERACTIONS•Monitor prothrombin time and International Normalized Ratio in patients concurrently administered ISTODAX (romidepsin) and warfarin sodium derivatives•Romidepsin is metabolized by CYP3A4◦Monitor patients for toxicity related to increased romidepsin exposure and follow dose modifications for toxicity when ISTODAX is initially co-administered with strong CYP3A4 inhibitors◦Avoid co-administration of ISTODAX (romidepsin) with rifampin and other potent inducers of CYP3A4•Exercise caution with concomitant use of ISTODAX and P-glycoprotein (P-gp, ABCB1) inhibitorsUSE IN SPECIFIC POPULATIONS•Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from ISTODAX, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother•Patients with moderate and severe hepatic impairment and/or patients with end-stage renal disease should be treated with caution
用药温馨提示:当您服用此药物时,需定期接受医疗专业人士的检查,以便随时针对其药效、副作用等情况进行监测。本网站所包含的信息旨在为患者提供帮助,不能代替医学建议和治疗。
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 罗米地辛冻干粉注射剂ISTODAX KIT FOR INJ 10MG
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 罗米地辛冻干粉注射剂ISTODAX KIT FOR INJ 10MG