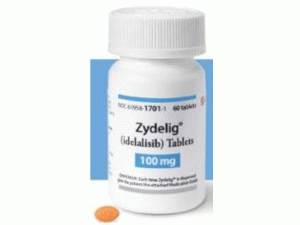
艾代拉里斯片Zydelig Tablets 150mg(Idelalisi)
 药店国别:
产地国家:美国
处方药:是
所属类别: 150毫克/片 60片/瓶
包装规格: 150毫克/片 60片/瓶
计价单位:瓶
生产厂家中文参考译名:
生产厂家英文名:Gilead Sciences
原产地英文商品名:Zydelig tab 150mg/tab 60tabs/box
原产地英文药品名:idelalisib
中文参考商品译名:Zydelig片 150毫克/片 60片/瓶
中文参考药品译名:艾代拉里斯
曾用名:Zydelig Tablets 100mg(Idelalisib 艾代拉里斯片)
简介:
近日,美国FDA批准Idelalisib(商品名:Zydelig)的三个适应症:和利妥昔单抗(Rituxan)联合治疗复发的慢性淋巴细胞白血病(CLL)、作为单药治疗复发性滤泡B细胞非霍奇金淋巴瘤(FL)和复发性小淋巴细胞淋巴瘤(SLL)。FDA对后两个适应症是加速批准,且患者之前都至少接受过两次全身治疗批准日期:2014年7月23日 公司:吉利德科学ZYDELIG®(艾代拉里斯[idelalisib])片剂,供口服使用最初的美国批准:2014年警告:致命和严重毒性:肝,重性腹泻,结肠炎,肺炎,感染和肠胃穿孔查看完整的盒装警告的完整处方信息。
•Zydelig治疗的患者中有16%至18%发生致命性和/或严重肝毒性。在治疗前和治疗期间监测肝功能。中断,然后减少或停止Zydelig。Zydelig治疗的患者中有14%至20%发生致命性和/或严重和严重腹泻或结肠炎。监测严重腹泻或结肠炎的发展。中断,然后减少或停止Zydelig。•4%的Zydeligtreated患者发生致命性和/或严重肺炎。监测肺部症状和双侧间质浸润。中断或停止Zydelig。•Zydelig治疗的患者中有21%至48%发生致命和/或严重感染。监测感染的体征和症状。如果怀疑有感染,则中断Zydelig。•临床试验中Zydeligtreated患者可能会发生严重肠道穿孔。如果怀疑肠穿孔,请停止Zydelig。
作用机制
Idelalisib是PI3K激酶的抑制剂,其在正常和恶性B细胞中表达。Idelalisib诱导细胞凋亡和抑制细胞增殖恶性B细胞和原发性肿瘤细胞中。Idelalisib抑制几种细胞信号传导包括B细胞受体(BCR)信号传导和CXCR4和CXCR5信号传导,其参与B细胞向淋巴结和淋巴结的运输和归巢骨髓。用idelalisib治疗淋巴瘤细胞导致抑制趋化性和粘附性,并降低细胞活力。
适应症和用法
Zydelig是一种激酶抑制剂,用于治疗以下患者:•复发性慢性淋巴细胞性白血病(CLL)联合利妥昔单抗应用于单用利妥昔单抗因其他合并症而被认为是适当治疗的患者。•已接受至少两种全身治疗的患者中的滤泡性B细胞非霍奇金淋巴瘤(FL)复发。•至少接受过两次全身治疗的患者的复发性小淋巴细胞性淋巴瘤(SLL)。
使用限制:
Zydelig未被指出,不建议用于任何患者的一线治疗。Zydelig未被指出,不建议与苯达莫司汀和/或利妥昔单抗联合用于治疗FL。总体而言,FL和SLL获得了加速批准反应速度。改善患者的生存或疾病相关症状尚未确定。继续批准这些适应证可能取决于临床受益的证实在验证性试验中。
剂量和给药
建议起始剂量:口服150毫克,每日两次。剂型和强度片剂:150mg,100mg。
禁忌症
严重过敏反应史,包括过敏反应和中毒性表皮坏死松解症。
警告和注意事项
•严重皮肤反应:监测患者发生严重皮肤反应并停止Zydelig。•过敏反应:监测患者的过敏反应并停止Zydelig。•嗜中性白血球减少症:监测血液计数。•胚胎-胎儿毒性:Zydelig可能会对胎儿造成伤害。建议妇女在服用Zydelig时对胎儿有潜在风险并避免怀孕。
不良反应
单药治疗Zydelig治疗的患者最常见的不良反应(发生率≥20%)为腹泻,疲劳,恶心,咳嗽,发热,腹痛,肺炎和皮疹。联合试验中Zydelig治疗患者最常见的不良反应(发生率≥30%)为腹泻,肺炎,发热,疲劳,皮疹,咳嗽和恶心。常见的实验室异常包括嗜中性白血球减少症,ALT升高和AST升高。
药物相互作用
•强效CYP3A抑制剂:如果没有其他疗法,则需要额外监测。•强CYP3A诱导剂:避免合用强效CYP3A诱导剂。•CYP3A底物:避免共同给药敏感的CYP3A底物。
在特定人群中使用哺乳期:
建议妇女不要母乳喂养。外上市情况美国:片剂150mg,100mg(2014.7.23);Gilead Sciences, Inc.公司持有;商品名Zydelig欧盟:片剂150mg,100mg(2014.9.18);Gilead Sciences International Ltd公司持有;商品名Zydelig临床优势Zydelig(idelalisib)是一种首创的高度选择性、口服有效的磷酸肌醇3-激酶delta(PI3K-delta)抑制剂。一项Ⅲ期临床试验(NCT01732913)显示在危险因素分层的所有亚组中,idelalisib组均显著延长了预估无进展生存期,idelalisib组患者总生存显著改善,并且具有显著更高的淋巴结反应率(93%、4%)。与利妥昔单抗单药治疗相比,艾代拉利司+利妥昔单抗联合疗法对研究的主要终点疾病无进展生存期具有显著的积极影响,表现出了高度统计学显著功效。
对于患有复发性血癌的人来说,它能够显著延长患者的无进展生存期。在一系列临床研究中显示出良好的治疗前景,且安全性及耐受性均较好。产品简介Idelalisib是首个上市的口服、选择性的磷酸肌醇3-激酶delta(PI3K-delta,P110-delta)抑制剂。P110-delta参与改变B淋巴细胞的免疫环境,对这类肿瘤细胞的活化、增殖、生存和迁移(trafficking)起着关键作用。Idelalisib的加速批准是基于一个单臂、多中心、开放标签的积极二期临床结果。
该临床实验招募了123位复发性的“惰性”非霍奇金淋巴瘤(iNHL)和小淋巴细胞淋巴瘤(SLL)患者。病人每天接受两次,每次150毫克艾代拉里斯治疗,一级实验终点是总应答率(ORR),二级实验终点是应答时间和无进展生存期。其中FL和SLL患者的总应答率分别为54%和58%。后者应答时间的中位数为11.9个月。这个结果和标准疗法的通常疗效相比相当或更好。艾代拉利司(Idelalisib)的获批上市,为慢性淋巴细胞白血病(CLL)的治疗在ibrutinib之后又带来一个新的选择。在美国,慢性淋巴细胞白血病(CLL)在成人白血病患者中人数排第二,预计2014年会增添超过15000名新患者。包括Idelalisib和ibrutinib在内的CLL新药研发,有望把CLL从死刑判决演变成一种可控制的慢性疾病。
英文版说明书
IMPORTANT SAFETY INFORMATIONWARNING:FATAL AND SERIOUS TOXICITIES: HEPATIC, SEVERE DIARRHEA, COLITIS, PNEUMONITIS, AND INTESTINAL PERFORATIONFatal and/or serious hepatotoxicity occurred in 14% of ZYDELIG-treated patients. Monitor hepatic function prior to and during treatment. Interrupt and then reduce or discontinue ZYDELIG as recommendedFatal and/or serious and severe diarrhea or colitis occurred in 14% of ZYDELIG-treated patients. Monitor for the development of severe diarrhea or colitis. Interrupt and then reduce or discontinue ZYDELIG as recommendedFatal and serious pneumonitis can occur. Monitor for pulmonary symptoms and bilateral interstitial infiltrates. Interrupt or discontinue ZYDELIG as recommendedFatal and serious intestinal perforation can occur in ZYDELIG-treated patients. Discontinue ZYDELIG for intestinal perforationContraindicationsHistory of serious allergic reactions, including anaphylaxis and toxic epidermal necrolysis (TEN)Warnings and PrecautionsHepatotoxicity: Findings were generally observed within the first 12 weeks of treatment and reversed with dose interruption. Upon rechallenge at a lower dose, ALT/AST elevations recurred in 26% of patients. In all patients, monitor ALT/AST every 2 weeks for the first 3 months, every 4 weeks for the next 3 months, and every 1 to 3 months thereafter. If ALT/AST is >3x upper limit of normal (ULN), monitor for liver toxicity weekly. If ALT/AST is >5x ULN, withhold ZYDELIG and monitor ALT/AST and total bilirubin weekly until resolved. Discontinue ZYDELIG for recurrent hepatotoxicity. Avoid concurrent use with other hepatotoxic drugsSevere diarrhea or colitis: Grade 3+ diarrhea can occur at any time and responds poorly to antimotility agents. Avoid concurrent use with other drugs that cause diarrheaPneumonitis: eva luate for pneumonitis in patients presenting with pulmonary symptoms such as cough, dyspnea, hypoxia, interstitial infiltrates on radiologic exam, or oxygen saturation decline by ≥5%Intestinal perforation: Advise patients to promptly report any new or worsening abdominal pain, chills, fever, nausea, or vomitingSevere cutaneous reactions: One case of TEN occurred in a study of ZYDELIG in combination with rituximab and bendamustine. Other severe or life-threatening (grade ≥3) cutaneous reactions have been reported. Monitor patients for the development of severe cutaneous reactions and discontinue ZYDELIG if a reaction occursAnaphylaxis: Serious allergic reactions including anaphylaxis have been reported. Discontinue ZYDELIG permanently and institute appropriate supportive measures if a reaction occursNeutropenia: Treatment-emergent grade 3-4 neutropenia occurred in 31% of ZYDELIG-treated patients in clinical trials. In all patients, monitor blood counts ≥every 2 weeks for the first 3 months. In patients with neutrophil counts <1.0 Gi/L, monitor weekly.Embryo-fetal toxicity: ZYDELIG may cause fetal harm. Women who are or become pregnant while taking ZYDELIG should be apprised of the potential hazard to the fetus. Advise women to avoid pregnancy while taking ZYDELIG and to use effective contraception during and at least 1 month after treatment with ZYDELIGAdverse ReactionsMost common adverse reactions (incidence ≥20%; all grades) in clinical studies, when used alone or in combination with rituximab, were diarrhea, pyrexia, fatigue, nausea, cough, pneumonia, abdominal pain, chills, and rashMost frequent serious adverse reactions (SAR) in clinical studies in combination with rituximab were pneumonia (17%), pyrexia (9%), sepsis (8%), febrile neutropenia (5%), and diarrhea (5%); SAR were reported in 49% of patients and 10% of patients discontinued due to adverse reactions. Most frequent SAR in clinical studies when used alone were pneumonia (15%), diarrhea (11%), and pyrexia (9%); SAR were reported in 50% of patients and 53% of patients discontinued or interrupted therapy due to adverse reactionsMost common lab abnormalities (incidence ≥30%; all grades) in clinical studies were neutropenia, hypertriglyceridemia, hyperglycemia, and ALT/AST elevationsDrug InteractionsCYP3A inducers: Avoid coadministration with strong CYP3A inducersCYP3A inhibitors: When coadministered with strong CYP3A inhibitors, monitor closely for ZYDELIG toxicityCYP3A substrates: Avoid coadministration with CYP3A substratesDosage and AdministrationAdult starting dose: One 150 mg tablet twice daily, swallowed whole with or without food. Continue treatment until disease progression or unacceptable toxicity. The safe dosing regimen for patients who require treatment longer than several months is unknownDose modification: Consult the ZYDELIG full Prescribing Information for dose modification and monitoring recommendations for the following specific toxicities: pneumonitis, ALT/AST elevations, bilirubin elevations, diarrhea, neutropenia, and thrombocytopenia. For other severe or life-threatening toxicities, withhold ZYDELIG until toxicity is resolved and reduce the dose to 100 mg, twice daily, upon resuming treatment. If severe or life-threatening toxicities recur upon rechallenge, ZYDELIG should be permanently discontinuedINDICATIONSZYDELIG is indicated for the treatment ofRelapsed follicular B-cell non-Hodgkin lymphoma (FL) in patients who have received at least two prior systemic therapiesRelapsed chronic lymphocytic leukemia (CLL) in combination with rituximab in patients for whom rituximab alone would be considered appropriate therapy due to other comorbiditiesRelapsed small lymphocytic lymphoma (SLL) in patients who have received at least two prior systemic therapiesThe FL and SLL indications were granted accelerated approval based on overall response rate; improvement in patient survival or disease-related symptoms has not been established
药店国别:
产地国家:美国
处方药:是
所属类别: 150毫克/片 60片/瓶
包装规格: 150毫克/片 60片/瓶
计价单位:瓶
生产厂家中文参考译名:
生产厂家英文名:Gilead Sciences
原产地英文商品名:Zydelig tab 150mg/tab 60tabs/box
原产地英文药品名:idelalisib
中文参考商品译名:Zydelig片 150毫克/片 60片/瓶
中文参考药品译名:艾代拉里斯
曾用名:Zydelig Tablets 100mg(Idelalisib 艾代拉里斯片)
简介:
近日,美国FDA批准Idelalisib(商品名:Zydelig)的三个适应症:和利妥昔单抗(Rituxan)联合治疗复发的慢性淋巴细胞白血病(CLL)、作为单药治疗复发性滤泡B细胞非霍奇金淋巴瘤(FL)和复发性小淋巴细胞淋巴瘤(SLL)。FDA对后两个适应症是加速批准,且患者之前都至少接受过两次全身治疗批准日期:2014年7月23日 公司:吉利德科学ZYDELIG®(艾代拉里斯[idelalisib])片剂,供口服使用最初的美国批准:2014年警告:致命和严重毒性:肝,重性腹泻,结肠炎,肺炎,感染和肠胃穿孔查看完整的盒装警告的完整处方信息。
•Zydelig治疗的患者中有16%至18%发生致命性和/或严重肝毒性。在治疗前和治疗期间监测肝功能。中断,然后减少或停止Zydelig。Zydelig治疗的患者中有14%至20%发生致命性和/或严重和严重腹泻或结肠炎。监测严重腹泻或结肠炎的发展。中断,然后减少或停止Zydelig。•4%的Zydeligtreated患者发生致命性和/或严重肺炎。监测肺部症状和双侧间质浸润。中断或停止Zydelig。•Zydelig治疗的患者中有21%至48%发生致命和/或严重感染。监测感染的体征和症状。如果怀疑有感染,则中断Zydelig。•临床试验中Zydeligtreated患者可能会发生严重肠道穿孔。如果怀疑肠穿孔,请停止Zydelig。
作用机制
Idelalisib是PI3K激酶的抑制剂,其在正常和恶性B细胞中表达。Idelalisib诱导细胞凋亡和抑制细胞增殖恶性B细胞和原发性肿瘤细胞中。Idelalisib抑制几种细胞信号传导包括B细胞受体(BCR)信号传导和CXCR4和CXCR5信号传导,其参与B细胞向淋巴结和淋巴结的运输和归巢骨髓。用idelalisib治疗淋巴瘤细胞导致抑制趋化性和粘附性,并降低细胞活力。
适应症和用法
Zydelig是一种激酶抑制剂,用于治疗以下患者:•复发性慢性淋巴细胞性白血病(CLL)联合利妥昔单抗应用于单用利妥昔单抗因其他合并症而被认为是适当治疗的患者。•已接受至少两种全身治疗的患者中的滤泡性B细胞非霍奇金淋巴瘤(FL)复发。•至少接受过两次全身治疗的患者的复发性小淋巴细胞性淋巴瘤(SLL)。
使用限制:
Zydelig未被指出,不建议用于任何患者的一线治疗。Zydelig未被指出,不建议与苯达莫司汀和/或利妥昔单抗联合用于治疗FL。总体而言,FL和SLL获得了加速批准反应速度。改善患者的生存或疾病相关症状尚未确定。继续批准这些适应证可能取决于临床受益的证实在验证性试验中。
剂量和给药
建议起始剂量:口服150毫克,每日两次。剂型和强度片剂:150mg,100mg。
禁忌症
严重过敏反应史,包括过敏反应和中毒性表皮坏死松解症。
警告和注意事项
•严重皮肤反应:监测患者发生严重皮肤反应并停止Zydelig。•过敏反应:监测患者的过敏反应并停止Zydelig。•嗜中性白血球减少症:监测血液计数。•胚胎-胎儿毒性:Zydelig可能会对胎儿造成伤害。建议妇女在服用Zydelig时对胎儿有潜在风险并避免怀孕。
不良反应
单药治疗Zydelig治疗的患者最常见的不良反应(发生率≥20%)为腹泻,疲劳,恶心,咳嗽,发热,腹痛,肺炎和皮疹。联合试验中Zydelig治疗患者最常见的不良反应(发生率≥30%)为腹泻,肺炎,发热,疲劳,皮疹,咳嗽和恶心。常见的实验室异常包括嗜中性白血球减少症,ALT升高和AST升高。
药物相互作用
•强效CYP3A抑制剂:如果没有其他疗法,则需要额外监测。•强CYP3A诱导剂:避免合用强效CYP3A诱导剂。•CYP3A底物:避免共同给药敏感的CYP3A底物。
在特定人群中使用哺乳期:
建议妇女不要母乳喂养。外上市情况美国:片剂150mg,100mg(2014.7.23);Gilead Sciences, Inc.公司持有;商品名Zydelig欧盟:片剂150mg,100mg(2014.9.18);Gilead Sciences International Ltd公司持有;商品名Zydelig临床优势Zydelig(idelalisib)是一种首创的高度选择性、口服有效的磷酸肌醇3-激酶delta(PI3K-delta)抑制剂。一项Ⅲ期临床试验(NCT01732913)显示在危险因素分层的所有亚组中,idelalisib组均显著延长了预估无进展生存期,idelalisib组患者总生存显著改善,并且具有显著更高的淋巴结反应率(93%、4%)。与利妥昔单抗单药治疗相比,艾代拉利司+利妥昔单抗联合疗法对研究的主要终点疾病无进展生存期具有显著的积极影响,表现出了高度统计学显著功效。
对于患有复发性血癌的人来说,它能够显著延长患者的无进展生存期。在一系列临床研究中显示出良好的治疗前景,且安全性及耐受性均较好。产品简介Idelalisib是首个上市的口服、选择性的磷酸肌醇3-激酶delta(PI3K-delta,P110-delta)抑制剂。P110-delta参与改变B淋巴细胞的免疫环境,对这类肿瘤细胞的活化、增殖、生存和迁移(trafficking)起着关键作用。Idelalisib的加速批准是基于一个单臂、多中心、开放标签的积极二期临床结果。
该临床实验招募了123位复发性的“惰性”非霍奇金淋巴瘤(iNHL)和小淋巴细胞淋巴瘤(SLL)患者。病人每天接受两次,每次150毫克艾代拉里斯治疗,一级实验终点是总应答率(ORR),二级实验终点是应答时间和无进展生存期。其中FL和SLL患者的总应答率分别为54%和58%。后者应答时间的中位数为11.9个月。这个结果和标准疗法的通常疗效相比相当或更好。艾代拉利司(Idelalisib)的获批上市,为慢性淋巴细胞白血病(CLL)的治疗在ibrutinib之后又带来一个新的选择。在美国,慢性淋巴细胞白血病(CLL)在成人白血病患者中人数排第二,预计2014年会增添超过15000名新患者。包括Idelalisib和ibrutinib在内的CLL新药研发,有望把CLL从死刑判决演变成一种可控制的慢性疾病。
英文版说明书
IMPORTANT SAFETY INFORMATIONWARNING:FATAL AND SERIOUS TOXICITIES: HEPATIC, SEVERE DIARRHEA, COLITIS, PNEUMONITIS, AND INTESTINAL PERFORATIONFatal and/or serious hepatotoxicity occurred in 14% of ZYDELIG-treated patients. Monitor hepatic function prior to and during treatment. Interrupt and then reduce or discontinue ZYDELIG as recommendedFatal and/or serious and severe diarrhea or colitis occurred in 14% of ZYDELIG-treated patients. Monitor for the development of severe diarrhea or colitis. Interrupt and then reduce or discontinue ZYDELIG as recommendedFatal and serious pneumonitis can occur. Monitor for pulmonary symptoms and bilateral interstitial infiltrates. Interrupt or discontinue ZYDELIG as recommendedFatal and serious intestinal perforation can occur in ZYDELIG-treated patients. Discontinue ZYDELIG for intestinal perforationContraindicationsHistory of serious allergic reactions, including anaphylaxis and toxic epidermal necrolysis (TEN)Warnings and PrecautionsHepatotoxicity: Findings were generally observed within the first 12 weeks of treatment and reversed with dose interruption. Upon rechallenge at a lower dose, ALT/AST elevations recurred in 26% of patients. In all patients, monitor ALT/AST every 2 weeks for the first 3 months, every 4 weeks for the next 3 months, and every 1 to 3 months thereafter. If ALT/AST is >3x upper limit of normal (ULN), monitor for liver toxicity weekly. If ALT/AST is >5x ULN, withhold ZYDELIG and monitor ALT/AST and total bilirubin weekly until resolved. Discontinue ZYDELIG for recurrent hepatotoxicity. Avoid concurrent use with other hepatotoxic drugsSevere diarrhea or colitis: Grade 3+ diarrhea can occur at any time and responds poorly to antimotility agents. Avoid concurrent use with other drugs that cause diarrheaPneumonitis: eva luate for pneumonitis in patients presenting with pulmonary symptoms such as cough, dyspnea, hypoxia, interstitial infiltrates on radiologic exam, or oxygen saturation decline by ≥5%Intestinal perforation: Advise patients to promptly report any new or worsening abdominal pain, chills, fever, nausea, or vomitingSevere cutaneous reactions: One case of TEN occurred in a study of ZYDELIG in combination with rituximab and bendamustine. Other severe or life-threatening (grade ≥3) cutaneous reactions have been reported. Monitor patients for the development of severe cutaneous reactions and discontinue ZYDELIG if a reaction occursAnaphylaxis: Serious allergic reactions including anaphylaxis have been reported. Discontinue ZYDELIG permanently and institute appropriate supportive measures if a reaction occursNeutropenia: Treatment-emergent grade 3-4 neutropenia occurred in 31% of ZYDELIG-treated patients in clinical trials. In all patients, monitor blood counts ≥every 2 weeks for the first 3 months. In patients with neutrophil counts <1.0 Gi/L, monitor weekly.Embryo-fetal toxicity: ZYDELIG may cause fetal harm. Women who are or become pregnant while taking ZYDELIG should be apprised of the potential hazard to the fetus. Advise women to avoid pregnancy while taking ZYDELIG and to use effective contraception during and at least 1 month after treatment with ZYDELIGAdverse ReactionsMost common adverse reactions (incidence ≥20%; all grades) in clinical studies, when used alone or in combination with rituximab, were diarrhea, pyrexia, fatigue, nausea, cough, pneumonia, abdominal pain, chills, and rashMost frequent serious adverse reactions (SAR) in clinical studies in combination with rituximab were pneumonia (17%), pyrexia (9%), sepsis (8%), febrile neutropenia (5%), and diarrhea (5%); SAR were reported in 49% of patients and 10% of patients discontinued due to adverse reactions. Most frequent SAR in clinical studies when used alone were pneumonia (15%), diarrhea (11%), and pyrexia (9%); SAR were reported in 50% of patients and 53% of patients discontinued or interrupted therapy due to adverse reactionsMost common lab abnormalities (incidence ≥30%; all grades) in clinical studies were neutropenia, hypertriglyceridemia, hyperglycemia, and ALT/AST elevationsDrug InteractionsCYP3A inducers: Avoid coadministration with strong CYP3A inducersCYP3A inhibitors: When coadministered with strong CYP3A inhibitors, monitor closely for ZYDELIG toxicityCYP3A substrates: Avoid coadministration with CYP3A substratesDosage and AdministrationAdult starting dose: One 150 mg tablet twice daily, swallowed whole with or without food. Continue treatment until disease progression or unacceptable toxicity. The safe dosing regimen for patients who require treatment longer than several months is unknownDose modification: Consult the ZYDELIG full Prescribing Information for dose modification and monitoring recommendations for the following specific toxicities: pneumonitis, ALT/AST elevations, bilirubin elevations, diarrhea, neutropenia, and thrombocytopenia. For other severe or life-threatening toxicities, withhold ZYDELIG until toxicity is resolved and reduce the dose to 100 mg, twice daily, upon resuming treatment. If severe or life-threatening toxicities recur upon rechallenge, ZYDELIG should be permanently discontinuedINDICATIONSZYDELIG is indicated for the treatment ofRelapsed follicular B-cell non-Hodgkin lymphoma (FL) in patients who have received at least two prior systemic therapiesRelapsed chronic lymphocytic leukemia (CLL) in combination with rituximab in patients for whom rituximab alone would be considered appropriate therapy due to other comorbiditiesRelapsed small lymphocytic lymphoma (SLL) in patients who have received at least two prior systemic therapiesThe FL and SLL indications were granted accelerated approval based on overall response rate; improvement in patient survival or disease-related symptoms has not been established
用药温馨提示:当您服用此药物时,需定期接受医疗专业人士的检查,以便随时针对其药效、副作用等情况进行监测。本网站所包含的信息旨在为患者提供帮助,不能代替医学建议和治疗。
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 艾代拉里斯片Zydelig Tablets 150mg(Idelalisi)
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 艾代拉里斯片Zydelig Tablets 150mg(Idelalisi)