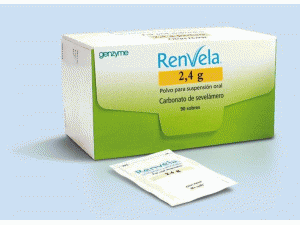
碳酸司维拉姆干混悬剂Sevelamer carbonate (Renvela 2.4g Pulver)
 产地国家:德国
处 方 药:是
所属类别:2.4克/包 90包/盒
包装规格:2.4克/包 90包/盒
计价单位:盒
生产厂家中文参考译名:健赞
生产厂家英文名:Genzyme
原产地英文商品名:RENVELA POWDER 2.4g/pack 90packs/box
原产地英文药品名:sevelamer carbonate
中文参考商品译名:RENVELA粉剂 2.4克/包 90包/盒
中文参考药品译名:碳酸司维拉姆
产地国家:德国
处 方 药:是
所属类别:2.4克/包 90包/盒
包装规格:2.4克/包 90包/盒
计价单位:盒
生产厂家中文参考译名:健赞
生产厂家英文名:Genzyme
原产地英文商品名:RENVELA POWDER 2.4g/pack 90packs/box
原产地英文药品名:sevelamer carbonate
中文参考商品译名:RENVELA粉剂 2.4克/包 90包/盒
中文参考药品译名:碳酸司维拉姆
简介
部份中文碳酸司维拉姆处方资料(仅供参股) 通用名:碳酸司维拉姆 商品名:Renvela 英文名:Sevelamer carbonate 剂型:干混悬剂、片剂。 规格:0.8g、2.4g、1.6g。 国内外上市情况 本品由美国健赞公司研发,目前司维拉姆已在美、日、加、与欧洲等共计40多国上市并广泛的使用。2007年10月19日,FDA批准碳酸司维拉姆片上市,2009年8月12日,批准碳酸司维拉姆口服干混悬剂上市,2009年8月,EMEA批准碳酸司维拉姆上市。 特点 司维拉姆作为现今唯一一种既不含钙、又不含有金属的磷酸盐结合剂。它不会为系统吸收,故能在提供安全、有效控制血清磷作用的同时还无钙和金属蓄积的担忧。司维拉姆尚有显著的低密度脂蛋白胆固醇水平降低的额外益处。 透析人群常见血清磷水平升高,会使心血管发病率和死亡率升高。因此控制血清磷浓度是必要的。碳酸司维拉姆不仅保留了盐酸司维拉姆的所有优点.且它还能同时提供碳酸盐缓冲剂的额外益处。一项直接临床比较证实碳酸司维拉姆和盐酸司维拉姆两药控制正行透析之慢性肾病人群血清磷浓度的疗效相当,但碳酸司维拉姆治疗个体更可能维持适宜的碳酸氢盐水平,故胃肠道副反应发生率更低。英文版说明书
Renvela®(sevelamer carbonate) is indicated for the control of serum phosphorus in patients with chronic kidney disease(CKD) on dialysis Renvela is contraindicated in patients with bowel obstruction Caution should be exercised in patients with dysphagia, swallowing disorders, severe gastrointestinal (GI) motility disorders including severe constipation, or major GI tract surgery Uncommon cases of bowel obstruction and perforation have been reported Serum bicarbonate and chloride levels should be monitored Vitamins D, E, K (coagulation parameters), and folic acid levels should be monitored The most frequently occurring adverse reactions in a short-term study with sevelamer carbonate tablets were nausea and vomiting In a short-term study of sevelamer carbonate powder dosed three times daily, adverse events were similar to those reported for sevelamer carbonate tablets In long-term studies with sevelamer hydrochloride, which contains the same active moiety as sevelamer carbonate, the most common adverse events included: vomiting, nausea, diarrhea, dyspepsia, abdominal pain, flatulence, and constipation Cases of fecal impaction and, less commonly, ileus, bowel obstruction, and bowel perforation have been reported Drug-drug interactions may occur with some medications and should be taken into consideration when instructing patients how to take Renvela Patients should be informed to take Renvela with meals and to adhere to their prescribed diets Please see accompanying full Prescribing Information. Renagel® (sevelamer hydrochloride) is indicated for the control of serum phosphorus in patients with chronic kidney disease (CKD) on dialysis Renagel is contraindicated in patients with hypophosphatemia or bowel obstruction Caution should be exercised in patients with dysphagia, swallowing disorders, severe gastrointestinal (GI) motility disorders including severe constipation or major GI tract surgery Common adverse events reported with Renagel include vomiting, nausea, diarrhea, dyspepsia, abdominal pain, and constipation Other events reported include pruritus, rash, fecal impaction, and intestinal obstruction Drug-drug interactions may occur with some medications and should be taken into consideration when instructing patients how to take Renagel Patients should be informed to take Renagel with meals and to adhere to their prescribed diets.用药温馨提示:当您服用此药物时,需定期接受医疗专业人士的检查,以便随时针对其药效、副作用等情况进行监测。本网站所包含的信息旨在为患者提供帮助,不能代替医学建议和治疗。
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 碳酸司维拉姆干混悬剂Sevelamer carbonate (Renvela 2.4g Pulver)
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 碳酸司维拉姆干混悬剂Sevelamer carbonate (Renvela 2.4g Pulver)