阿米卡星脂质体吸入悬浮液 说明书
产地国家:美国 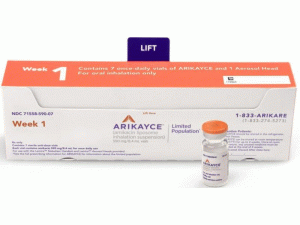 处 方 药:是
所属类别:590毫克/8.4毫升 10毫升/瓶 28瓶/套件
包装规格:590毫克/8.4毫升 10毫升/瓶 28瓶/套件
计价单位:套件
生产厂家中文参考译名:PARI Respiratory Equipment Inc.
生产厂家英文名:PARI Respiratory Equipment Inc.
原产地英文商品名:ARIKAYCE inhalation suspension kit 590mg/8.4ml 10nl/vial 28vial/box
原产地英文药品名:amikacin liposome
中文参考商品译名:ARIKAYCE吸入悬浮液 590毫克/8.4毫升 10毫升/瓶 28瓶/套件
中文参考药品译名:阿米卡星脂质体
处 方 药:是
所属类别:590毫克/8.4毫升 10毫升/瓶 28瓶/套件
包装规格:590毫克/8.4毫升 10毫升/瓶 28瓶/套件
计价单位:套件
生产厂家中文参考译名:PARI Respiratory Equipment Inc.
生产厂家英文名:PARI Respiratory Equipment Inc.
原产地英文商品名:ARIKAYCE inhalation suspension kit 590mg/8.4ml 10nl/vial 28vial/box
原产地英文药品名:amikacin liposome
中文参考商品译名:ARIKAYCE吸入悬浮液 590毫克/8.4毫升 10毫升/瓶 28瓶/套件
中文参考药品译名:阿米卡星脂质体
 处 方 药:是
所属类别:590毫克/8.4毫升 10毫升/瓶 28瓶/套件
包装规格:590毫克/8.4毫升 10毫升/瓶 28瓶/套件
计价单位:套件
生产厂家中文参考译名:PARI Respiratory Equipment Inc.
生产厂家英文名:PARI Respiratory Equipment Inc.
原产地英文商品名:ARIKAYCE inhalation suspension kit 590mg/8.4ml 10nl/vial 28vial/box
原产地英文药品名:amikacin liposome
中文参考商品译名:ARIKAYCE吸入悬浮液 590毫克/8.4毫升 10毫升/瓶 28瓶/套件
中文参考药品译名:阿米卡星脂质体
处 方 药:是
所属类别:590毫克/8.4毫升 10毫升/瓶 28瓶/套件
包装规格:590毫克/8.4毫升 10毫升/瓶 28瓶/套件
计价单位:套件
生产厂家中文参考译名:PARI Respiratory Equipment Inc.
生产厂家英文名:PARI Respiratory Equipment Inc.
原产地英文商品名:ARIKAYCE inhalation suspension kit 590mg/8.4ml 10nl/vial 28vial/box
原产地英文药品名:amikacin liposome
中文参考商品译名:ARIKAYCE吸入悬浮液 590毫克/8.4毫升 10毫升/瓶 28瓶/套件
中文参考药品译名:阿米卡星脂质体
简介
近日,美国食品药品监督管理局(FDA)批准ARIKAYCE(amikacin liposome,中文译名:阿米卡星脂质体)吸入混悬液上市,用于治疗鸟分枝杆菌复合物(MAC)肺部疾病,这是针对成年患者的联合抗菌药物治疗方案的一部分,这些患者的治疗选择有限或没有其他选择。 ARIKAYCE是在美国获批的首个也是唯一的一种疗法,专门用于患有MAC肺疾病的患者,MAC肺疾病是一种慢性衰弱性疾病,可显着增加患者的发病率和死亡率。 ARIKAYCE是首个通过“有限人群抗细菌和抗真菌药物”(LPAD)批准的产品。LPAD是《 21世纪治愈法案》的一部分,旨在促进开发新的抗菌药物,以治疗需求有限的有限人群中的严重或威胁生命的感染。 批准日期:2018年10月3日 公司:PARI制药公司 ARIKAYCE(阿米卡星脂质体[amikacin liposome])吸入悬浮液,用于口服吸入 美国初次批准:2018年 警告:呼吸不良的风险增加反应 有关完整的框内警告,请参阅完整的处方信息。 ARIKAYCE与呼吸不良反应增加的风险相关,包括过敏性肺炎,咯血,支气管痉挛和潜在的肺部疾病加重,在某些情况下导致了住院治疗。 作用机理 ARIKAYCE是一种抗菌药物[请参见微生物学]。 适应症和用途 人口有限:ARIKAYCE是一种氨基糖苷类抗菌药物,适用于仅有有限治疗选择或没有其他治疗选择的成年人,用于治疗鸟分枝杆菌复合物(MAC)肺病,作为联合抗菌药物治疗方案的一部分,如果患者至少经过6次痰培养仍未达到阴性连续数月的多药背景方案治疗。由于目前仅可获得ARIKAYCE的有限临床安全性和有效性数据,因此请保留ARIKAYCE以便用于治疗选择有限或没有其他选择的成年人。该药物被指定用于有限和特定人群 该适应症是根据第6个月实现痰培养物转化(定义为连续3个月阴性痰培养)而在加速批准下批准的。尚未确定临床获益。 使用限制 ARIKAYCE仅在难治性MAC肺病患者中进行了研究,难治性MAC肺病定义为在至少连续6个月接受多药背景疗法后,痰培养阴性的患者。对于非难治性MAC肺部疾病的患者,不建议使用ARIKAYCE。 剂量和给药 •仅用于口服吸入。 •仅将ARIKAYCE样品瓶与Lamira雾化器系统一起使用。 •成人的推荐剂量是每天口服一次吸入一个590mg/8.4mL ARIKAYCE小瓶的内容物。 •有气道高反应病史的患者应考虑使用吸入性支气管扩张剂进行预处理。 剂量形式和强度 ARIKAYCE以无菌水性脂质体悬浮液的形式提供,可在含阿米卡星590mg/8.4mL的单位剂量玻璃小瓶中口服吸入。 禁忌症 已知对任何氨基糖苷过敏的患者禁用ARIKAYCE。 警告和注意事项 •超敏性肺炎:有ARIKAYCE治疗报告;如果发生超敏性肺炎,应终止ARIKAYCE并在医学上适当地管理患者。 •咯血:据报道,ARIKAYCE治疗使咯血的频率更高。如果发生咯血,请按病历适当处理患者。 •支气管痉挛:据报道,ARIKAYCE治疗支气管痉挛的发生率更高。如果在ARIKAYCE治疗期间发生这种情况,请以医学上适当的方式对待患者。 •基础肺部疾病加重:据报道,ARIKAYCE治疗使基础肺部疾病加重的频率更高。如果在ARIKAYCE治疗期间发生这种情况,请以医学上适当的方式对待患者。 •耳毒性:据报道,ARIKAYCE治疗的耳毒性发生率更高。密切监视已知或怀疑听觉或前庭功能障碍的患者。如果患者出现耳鸣,则可能会出现耳毒性的早期症状。 •肾毒性:氨基糖苷类可能引起肾毒性。处方ARIKAYCE时,可能需要对已知或怀疑肾功能不全的患者进行密切监测。 •神经肌肉阻滞:由于对神经肌肉功能有潜在的咖喱样作用,因此氨基糖苷类可能会加重肌肉虚弱。如果发生神经肌肉阻滞,可以通过服用钙盐逆转,但可能需要机械辅助。 •胚胎-胎儿毒性:氨基糖苷类可能导致暴露于子宫内的小儿患者完全,不可逆的双侧先天性耳聋。 不良反应 难治性MAC肺部疾病患者最常见的不良反应(发生率≥10%,高于对照组)是:言语障碍,咳嗽,支气管痉挛,咯血,耳毒性,上呼吸道刺激,肌肉骨骼疼痛,疲劳/乏力和基础肺病加重,腹泻和恶心。 包装供应/存储和处理方式 供应方式 590mg/8.4mL ARIKAYCE(阿米卡星脂质体吸入悬浮液)装在无菌,单位剂量的10mL玻璃小瓶中。 该产品以28小瓶的形式分发。 每个纸箱包含28天的药品供应量(28个小瓶)。 除了纸箱中的ARIKAYCE小瓶外,还提供了一个Lamira雾化器手机和四个Lamira雾化器头。 NDC 71558-590-28 Lamira雾化器系统包含一个控制器,一个备用气雾头,一个备用听筒,电源线和配件。 储存和处理 将ARIKAYCE小瓶在2°C至8°C(36°F至46°F)冷藏保存,直到小瓶到期为止。不要冻结。过期后,请丢弃所有未使用的药物。 ARIKAYCE可以在最高25°C(77°F)的室温下保存4周。 一次在房间里温度下,任何未使用的药物必须在4周结束时丢弃。 提示:订购本品请提前二周半!英文版说明书
FDA Approval of ARIKAYCE® (amikacin liposome inhalation suspension), the First and Only Therapy Specifically Indicated for the Treatment of Mycobacterium Avium Complex (MAC) Lung Disease in Adult Patients with Limited or No Alternative Treatment Options U.S. Food and Drug Administration (FDA) has granted accelerated approval of ARIKAYCE® (amikacin liposome inhalation suspension) for the treatment of Mycobacterium avium complex(MAC)lung disease as part of a combination antibacterial drug regimen for adult patients who have limited or no alternative treatment options. ARIKAYCE is the first and only therapy approved in the U.S. specifically for patients with MAC lung disease, a chronic and debilitating condition that can significantly increase patient morbidity and mortality. ARIKAYCE is the first product approved via the Limited Population Pathway for Antibacterial and Antifungal Drugs (LPAD). LPAD, which was enacted as part of the 21st Century Cures Act, serves to advance the development of new antibacterial drugs to treat serious or life-threatening infections in limited populations of patients with unmet needs. About ARIKAYCE® (amikacin liposome inhalation suspension) ARIKAYCE is the first and only FDA-approved therapy indicated for the treatment of Mycobacterium avium complex (MAC) lung disease as part of a combination antibacterial drug regimen for adult patients with limited or no alternative treatment options. ARIKAYCE is a novel, inhaled, once-daily formulation of amikacin, an established antibiotic that was historically administered intravenously and associated with severe toxicity to hearing, balance, and kidney function. Insmed’s proprietary PULMOVANCE™ liposomal technology enables the delivery of amikacin directly to the lungs, where it is taken up by lung macrophages where the infection resides. This approach prolongs the release of amikacin in the lungs while limiting systemic exposure. ARIKAYCE is administered once daily using the Lamira™ Nebulizer System manufactured by PARI Pharma GmbH. About PARI Pharma and the Lamira™ Nebulizer System ARIKAYCE® (amikacin liposome inhalation suspension) is delivered by a novel inhalation device, the Lamira™ Nebulizer System, developed by PARI. Lamira™ is a quiet, portable nebulizer that enables efficient aerosolization of liquid medications, including liposomal formulations such as ARIKAYCE, via a vibrating, perforated membrane. Based on PARI's 100-year history working with aerosols, PARI is dedicated to advancing inhalation therapies by developing innovative delivery platforms and new pharmaceutical formulations that work together to improve patient care. About CONVERT (INS-212) and INS-312 CONVERT is a randomized, open-label, global Phase 3 trial designed to confirm the sputum culture conversion results seen in Insmed's Phase 2 clinical trial of ARIKAYCE in patients with refractory NTM lung disease caused by MAC. CONVERT is being conducted in 18 countries at more than 125 sites. The primary efficacy endpoint is the proportion of patients who achieved sputum culture conversion at Month 6 in the ARIKAYCE plus GBT arm compared to the GBT-only arm. Patients who achieved sputum culture conversion by Month 6 are continuing in the CONVERT study for an additional 12 months of treatment following the first monthly negative sputum culture. Patients who did not culture convert may have been eligible to enroll in our INS-312 study. INS-312 is a single-arm open-label extension study for patients who completed six months of treatment in the INS-212 study but did not demonstrate culture conversion by Month 6. Under the study protocol, non-converting patients in the ARIKAYCE plus GBT arm of the INS-212 study will receive an additional 12 months of ARIKAYCE plus GBT. Patients who crossed over from the GBT-only arm of the INS-212 study will receive 12 months of treatment of ARIKAYCE plus GBT. About ARIKAYCE® (amikacin liposome inhalation suspension) ARIKAYCE is the first and only FDA-approved therapy indicated for the treatment of Mycobacterium avium complex (MAC) lung disease as part of a combination antibacterial drug regimen for adult patients with limited or no alternative treatment options. ARIKAYCE is a novel, inhaled, once-daily formulation of amikacin, an established antibiotic that was historically administered intravenously and associated with severe toxicity to hearing, balance, and kidney function. Insmed’s proprietary PULMOVANCE™ liposomal technology enables the delivery of amikacin directly to the lungs, where it is taken up by lung macrophages where the infection resides. This approach prolongs the release of amikacin in the lungs while limiting systemic exposure. ARIKAYCE is administered once daily using the Lamira™ Nebulizer System manufactured by PARI Pharma GmbH. About PARI Pharma and the Lamira™ Nebulizer System ARIKAYCE® (amikacin liposome inhalation suspension) is delivered by a novel inhalation device, the Lamira™ Nebulizer System, developed by PARI. Lamira™ is a quiet, portable nebulizer that enables efficient aerosolization of liquid medications, including liposomal formulations such as ARIKAYCE, via a vibrating, perforated membrane. Based on PARI's 100-year history working with aerosols, PARI is dedicated to advancing inhalation therapies by developing innovative delivery platforms and new pharmaceutical formulations that work together to improve patient care. About CONVERT (INS-212) and INS-312 CONVERT is a randomized, open-label, global Phase 3 trial designed to confirm the sputum culture conversion results seen in Insmed's Phase 2 clinical trial of ARIKAYCE in patients with refractory NTM lung disease caused by MAC. CONVERT is being conducted in 18 countries at more than 125 sites. The primary efficacy endpoint is the proportion of patients who achieved sputum culture conversion at Month 6 in the ARIKAYCE plus GBT arm compared to the GBT-only arm. Patients who achieved sputum culture conversion by Month 6 are continuing in the CONVERT study for an additional 12 months of treatment following the first monthly negative sputum culture. Patients who did not culture convert may have been eligible to enroll in our INS-312 study. INS-312 is a single-arm open-label extension study for patients who completed six months of treatment in the INS-212 study but did not demonstrate culture conversion by Month 6. Under the study protocol, non-converting patients in the ARIKAYCE plus GBT arm of the INS-212 study will receive an additional 12 months of ARIKAYCE plus GBT. Patients who crossed over from the GBT-only arm of the INS-212 study will receive 12 months of treatment of ARIKAYCE plus GBT.用药温馨提示:当您服用此药物时,需定期接受医疗专业人士的检查,以便随时针对其药效、副作用等情况进行监测。本网站所包含的信息旨在为患者提供帮助,不能代替医学建议和治疗。
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 阿米卡星脂质体吸入悬浮液 说明书
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 阿米卡星脂质体吸入悬浮液 说明书