人血白蛋白Albumin DS(Albumin Human)
 药店国别:
产地国家:德国
处方药:是
所属类别: 25% 50毫升/瓶
包装规格: 25% 50毫升/瓶
计价单位:瓶
生产厂家中文参考译名:
生产厂家英文名:Behring
原产地英文商品名:Albumin DS 25% VL 50ML
原产地英文药品名:Albumin Human
中文参考商品译名:人血白蛋白 25% 50毫升瓶
中文参考药品译名:人血白蛋白
药店国别:
产地国家:德国
处方药:是
所属类别: 25% 50毫升/瓶
包装规格: 25% 50毫升/瓶
计价单位:瓶
生产厂家中文参考译名:
生产厂家英文名:Behring
原产地英文商品名:Albumin DS 25% VL 50ML
原产地英文药品名:Albumin Human
中文参考商品译名:人血白蛋白 25% 50毫升瓶
中文参考药品译名:人血白蛋白
简介
人血白蛋白25%注射液 -Grifols[Albutein inj制造商Grifols (Grifols)成份人血白蛋白 HumanAlbumin 适应症 低血容量性休克,长期接受血液透析患者的辅助用药,心肺搭桥过程中使用,ARDS,严重创伤或手术引起的白蛋白降低,某些急性肾病,急性肝衰竭或腹水,新生儿高胆红素血症。 用量成人 : 开始使用100 mL静脉输注,然后根据病情需要确定输入量和输注速度。 儿童 : 通常使用成人剂量的1/4-1/2,或2.4-4 mL/kg静脉输注。本品相容的稀释液为0.9%的氯化钠或5%葡萄糖。 禁忌 1.对白蛋白有严重过敏者。 2.高血压患者,急性心脏病者、正常血容量及高血容量的心力衰竭患者。 3.严重贫血患者。 4.肾功能不全者。 注意事项 1.药液呈现混浊、沉淀、异物或瓶子有裂纹、瓶盖松动、过期失效等情况不可使用。 2.本品开启后,应一次输注完毕,不得分次或给第二人输用。 3.输注过程中如发现病人有不适反应,应立即停止输用。 4.有明显脱水者应同时补液。 5.运输及贮存过程中严禁冻结。 孕妇及哺乳期妇女用药 对孕妇或可能怀孕妇女的用药应慎重,如有必要应用时,应在医师指导和严密观察下使用。 药物相互作用 勿与注射用水、含有蛋白质水解物或酒精的液体混合输注。 药物过量 因本品有高渗作用,过量注射时,可造成脱水、机体循环负荷增加、充血性心力衰竭和肺水肿。 不良反应 过敏性或热原性反应的主要症状是发烧和寒战 ;偶见皮疹,恶心,呕吐,心悸,低血压。英文版说明书
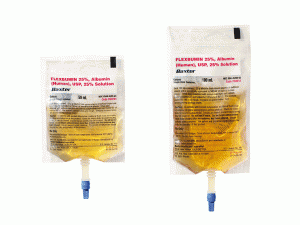
Indications & Usage FLEXBUMIN 5%, Albumin (Human), USP, 5% SolutionFLEXBUMIN 5% [Albumin (Human)] 5% Solution is a sterile, nonpyrogenic preparation of albumin in a single dosage form for intravenous administration.FLEXBUMIN 5% is indicated for hypovolemia, hypoalbuminemia due to general causes, burns, and for use during cardiopulmonary bypass surgery as a component of the pump prime.Indications & Usage FLEXBUMIN 25%, Albumin (Human), USP, 25% SolutionFLEXBUMIN 25% [Albumin (Human)] 25% Solution is a sterile, nonpyrogenic preparation of albumin in a single dosage form for intravenous administration.FLEXBUMIN 25% is indicated for hypovolemia, hypoalbuminemia due to general causes, burns, adult respiratory distress syndrome (ARDS), and nephrosis, and for use during cardiopulmonary bypass surgery as a component of the pump prime, and hemolytic disease of the newborn (HDN).Important Risk Information for FLEXBUMIN 5%•FLEXBUMIN 5% is contraindicated in patients with a history of allergic reactions to human albumin and any of the excipients, severe anemia, and heart failure.•Do not use if turbid. Do not begin administration more than 4 hours after the container has been entered.•Do not dilute with Sterile Water for injection as this can cause hemolysis. There exists a risk of potentially fatal hemolysis and acute renal failure from the use of Sterile Water for Injection as a diluent for Albumin (Human). Acceptable diluents include 0.9% Sodium Chloride or 5% Dextrose in Water.•FLEXBUMIN 5% is made from human blood. It carries an extremely remote risk for transmission of viral diseases and variant Creutzfeldt-Jakob disease (vCJD) and theoretical risk for transmission of Creutzfeldt-Jakob disease (CJD).•FLEXBUMIN 5% must be administered intravenously. FLEXBUMIN 5% should be administered slowly (5 to 10 mL/min) to avoid too rapid a rise in the blood pressure. More rapid administration can cause circulatory overload and pulmonary edema.•Monitor blood pressure in trauma patients and postoperative patients resuscitated with FLEXBUMIN 5% in order to detect rebleeding secondary to clot disruption.•In post marketing experience anaphylactic shock, anaphylactic reaction, hypersensitivity/allergic reactions, headache, dysgeusia, myocardial infarction, atrial fibrillation, tachycardia, hypotension, flushing, pulmonary edema, dyspnea, vomiting, nausea, urticaria, rash, pruritus, pyrexia and chills have been reported.CAUTION: : Do not use plastic containers in series connections. Such use could result in air embolism due to residual air being drawn from the primary container before the administration of the fluid from the secondary container is complete.Please see the full Prescribing Information for FLEXBUMIN 5%.Important Risk Information for FLEXBUMIN 25%•FLEXBUMIN 25% is contraindicated in patients with a history of allergic reactions to human albumin and any of the excipients, severe anemia, and heart failure.•Do not use if turbid. Do not begin administration more than 4 hours after the container has been entered.•Do not dilute with Sterile Water for injection as this can cause hemolysis. There exists a risk of potentially fatal hemolysis and acute renal failure from the use of Sterile Water for Injection as a diluent for Albumin (Human) in concentrations of 20% or higher. Acceptable diluents include 0.9% Sodium Chloride or 5% Dextrose in Water.•FLEXBUMIN 25% is made from human blood. It carries an extremely remote risk for transmission of viral diseases and variant Creutzfeldt-Jakob disease (vCJD) and theoretical risk for transmission of Creutzfeldt-Jakob disease (CJD).•FLEXBUMIN 25% must be administered intravenously at a rate not to exceed 1 mL/min to patients with normal blood volume. More rapid administration can cause circulatory overload and pulmonary edema.•Monitor blood pressure in trauma patients and postoperative surgery patients resuscitated with FLEXBUMIN 25% in order to detect rebleeding secondary to clot disruption.•In post marketing experience anaphylactic shock, anaphylactic reaction, hypersensitivity/allergic reactions, headache, dysgeusia, myocardial infarction, atrial fibrillation, tachycardia, hypotension, flushing, pulmonary edema, dyspnea, vomiting, nausea, urticaria, rash, pruritus, pyrexia and chills have been reported.CAUTION: Do not use plastic containers in series connections. Such use could result in air embolism due to residual air being drawn from the primary container before the administration of the fluid from the secondary container is complete.用药温馨提示:当您服用此药物时,需定期接受医疗专业人士的检查,以便随时针对其药效、副作用等情况进行监测。本网站所包含的信息旨在为患者提供帮助,不能代替医学建议和治疗。
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 人血白蛋白Albumin DS(Albumin Human)
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 人血白蛋白Albumin DS(Albumin Human)