卡赞替尼片Cabometyx Tablets 60mg(cabozantinib)
 药店国别:
产地国家:美国
处方药:是
所属类别: 60毫克/片 30片/瓶
包装规格: 60毫克/片 30片/瓶
计价单位:瓶
生产厂家中文参考译名:
生产厂家英文名:Exelixis,Inc
原产地英文商品名:Cabometyx 60mg/Tablets 30Tablets/bottle
原产地英文药品名:cabozantinib
中文参考商品译名:Cabometyx片 60毫克/片 30片/瓶
中文参考药品译名:卡赞替尼
曾用名:
简介:
近日,美国食品和药物管理局(FDA)扩大批准CABOMETYX(cabozantinib 中文译名:卡赞替尼)片剂,用于肝细胞癌(HCC)患者,这些患者之前曾接受过索拉非尼治疗。 HCC是最常见的肝癌形式,也是美国癌症相关死亡增长最快的原因。对于患有这种侵袭性肝癌的患者来说,CABOMETYX的这一新指征是一项重要的治疗进展,这是一个需要新治疗选择,”Exelixis总裁兼首席执行官Michael M. Morrissey博士说。 “这一批准是一个重要的里程碑。批准日期:2016年4月25日 公司:Exelixis,IncCABOMETYX(卡赞替尼[cabozantinib])片剂,口服使用美国最初批准:2012年
作用机制体
外生化和/或细胞试验表明,cabozantinib抑制MET,VEGFR-1,-2和-3,AXL,RET,ROS1,TYRO3,MER,KIT,TRKB,FLT-3和TIE的酪氨酸激酶活性-2。这些受体酪氨酸激酶参与正常细胞功能和病理过程,例如肿瘤发生,转移肿瘤血管生成,药物抗性和肿瘤微环境的维持。
适应症和用法
CABOMETYX是一种激酶抑制剂,适用于治疗•晚期肾细胞癌(RCC)患者。•曾接受过索拉非尼治疗的肝细胞癌(HCC)患者。
剂量和给药
•推荐剂量:口服60毫克,每日一次。•在进食前至少1小时或至少2小时后给药。•切勿用卡博替尼胶囊替代CABOMETYX片剂。
剂量形式和强度
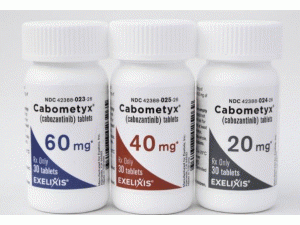
片剂:20mg,40mg和60mg。
禁忌症
没有。
警告和注意事项
•出血:如果有近期出血史,请勿给予CABOMETYX。•穿孔和瘘管:监测症状。因无法控制的瘘管或胃肠穿孔而停止使用CABOMETYX。•血栓形成事件:停止CABOMETYX用于心肌梗塞,脑梗塞或其他严重的血栓栓塞事件。•高血压和高血压危机:定期监测血压。用抗高血压治疗未充分控制的高血压中断。
停止CABOMETYX治疗无法控制的高血压危象或严重高血压抗高血压治疗。•腹泻:可能很严重。中断CABOMETYX立即降低腹泻,结果或降至等级。推荐标准的腹泻治疗。•手掌足底红斑感觉(PPE):中断CABOMETYX治疗直至PPE结算或降至等级。•蛋白尿:监测尿蛋白。停止肾病综合症。•下颌骨坏死:在进行侵入性牙科手术和开发ONJ之前,至少保留CABOMETYX至少28天。•伤口并发症:扣留CABOMETYX用于需要医疗干预的裂开或并发症。•可逆性后部白质脑病综合征(RPLS):停止使用CABOMETYX。•胚胎 - 胎儿毒性:可能导致胎儿伤害。建议女性有可能对胎儿造成潜在风险,并在治疗期间和最后一次给药后4个月内使用有效的感染。
不良反应
最常见(≥25%)的不良反应是:腹泻,疲劳,皮肤萎缩,手掌-足底红斑感觉(PPE),恶心,高血压和呕吐。
药物相互作用
•强CYP3A4抑制剂:如果无法避免给药,则减少CABOMETYX剂量。•强CYP3A4诱导剂:如果无法避免给药,则增加CABOMETYX剂量。
用于特定人群
•肝功能损害:减少中度肝功能损害患者的CABOMETYX剂量。避免患有严重肝病的患者损害。•哺乳期:建议不要母乳喂养。
包装提供/存储和处理
CABOMETYX片剂供货如下:60mg片剂是黄色薄膜包衣,椭圆形,没有刻痕,在一侧用“XL”压印,在另一侧用“60”压印; 有30片装:NDC 42388-023-2640mg片剂是黄色薄膜包衣,三角形没有刻痕,在一侧用“XL”压印,在另一侧用“40”压印; 有30片装:NDC 42388-025-2620mg片剂是黄色薄膜包衣,圆形没有刻痕,在一侧用“XL”压印,在另一侧用“20”压印; 有30片装:NDC 42388-024-26将CABOMETYX储存在20°C至25°C(68°F至77°F); 允许从15°C到30°C(59°F至86°F)[见USP受控室温]。
英文版说明书
Food and Drug Administration approved Cabometyx (cabozantinib) tablets for the treatment of patients with hepatocellular carcinoma (HCC) who have been previously treated with Nexavar (sorafenib).The Food and Drug Administration (FDA) approved Cabometyx (cabozantinib) tablets for the treatment of patients with hepatocellular carcinoma (HCC) who have been previously treated with Nexavar (sorafenib), according to Exelixis, the drug’s manufacturer.“This new indication for CABOMETYX is an important treatment advance for patients with this aggressive form of liver cancer, a community in need of new therapeutic options,” said Michael M. Morrissey, Ph.D., president and chief executive officer of Exelixis, said in a press release.The agency based its approval on results from the pivotal phase 3 CELESTIAL trial – designed to eva luate Cabometyx compared with placebo in 707 patients with advanced HCC, which is the most common form of liver cancer and the fastest-rising cause of cancer-related death in the U.S., according to the release.In the randomized, double-blind, placebo-controlled study, patients were randomized to receive 60 mg of Cabometyx daily (470 patients) or placebo (237 patients).Overall survival served as the primary endpoint, while secondary endpoints included objective response rate and progression-free survival – or the time until disease progression or worsening.Patients treated with Cabometyx demonstrated superior overall survival compared with placebo (10.2 vs. 8months), and median progression-free survival was more than double (5.2 vs. 1.9 months) compared with placebo.Four percent of percent of patients in the Cabometyx arm experienced an objective response rate compared with .04 percent of those who received placebo. In addition, disease control (partial response or stable disease) was achieved by 64 percent compared with 33 percent, respectively.The safety profile of Cabometyx appeared consistent with previous findings. The most common grade 3 or 4 side effects in the treatment arm included hand-foot syndrome (17 percent vs. 0 percent), hypertension (16 percent vs. 2 percent), increased aspartate aminotransferase (12 percent vs. 7 percent), fatigue (10 percent vs. 4 percent) and diarrhea (10 percent vs. 2 percent) compared with placebo. Sixteen percent of patients in the Cabometyx arm and three percent of patients in the placebo arm discontinued treatment due to treatment-related side effects.“Patients with this form of advanced liver cancer have few treatment options, particularly once their disease progresses following treatment with sorafenib,” lead investigator Ghassan K. Abou-Alfa, M.D., from Memorial Sloan Kettering Cancer Center, said in the release. “Physicians are eager for new options for these patients, and the results of the CELESTIAL trial demonstrate that Cabometyx has the efficacy and safety profile to become an important new therapy in our efforts to slow disease progression and improve treatment outcomes.”
药店国别:
产地国家:美国
处方药:是
所属类别: 60毫克/片 30片/瓶
包装规格: 60毫克/片 30片/瓶
计价单位:瓶
生产厂家中文参考译名:
生产厂家英文名:Exelixis,Inc
原产地英文商品名:Cabometyx 60mg/Tablets 30Tablets/bottle
原产地英文药品名:cabozantinib
中文参考商品译名:Cabometyx片 60毫克/片 30片/瓶
中文参考药品译名:卡赞替尼
曾用名:
简介:
近日,美国食品和药物管理局(FDA)扩大批准CABOMETYX(cabozantinib 中文译名:卡赞替尼)片剂,用于肝细胞癌(HCC)患者,这些患者之前曾接受过索拉非尼治疗。 HCC是最常见的肝癌形式,也是美国癌症相关死亡增长最快的原因。对于患有这种侵袭性肝癌的患者来说,CABOMETYX的这一新指征是一项重要的治疗进展,这是一个需要新治疗选择,”Exelixis总裁兼首席执行官Michael M. Morrissey博士说。 “这一批准是一个重要的里程碑。批准日期:2016年4月25日 公司:Exelixis,IncCABOMETYX(卡赞替尼[cabozantinib])片剂,口服使用美国最初批准:2012年
作用机制体
外生化和/或细胞试验表明,cabozantinib抑制MET,VEGFR-1,-2和-3,AXL,RET,ROS1,TYRO3,MER,KIT,TRKB,FLT-3和TIE的酪氨酸激酶活性-2。这些受体酪氨酸激酶参与正常细胞功能和病理过程,例如肿瘤发生,转移肿瘤血管生成,药物抗性和肿瘤微环境的维持。
适应症和用法
CABOMETYX是一种激酶抑制剂,适用于治疗•晚期肾细胞癌(RCC)患者。•曾接受过索拉非尼治疗的肝细胞癌(HCC)患者。
剂量和给药
•推荐剂量:口服60毫克,每日一次。•在进食前至少1小时或至少2小时后给药。•切勿用卡博替尼胶囊替代CABOMETYX片剂。
剂量形式和强度
片剂:20mg,40mg和60mg。
禁忌症
没有。
警告和注意事项
•出血:如果有近期出血史,请勿给予CABOMETYX。•穿孔和瘘管:监测症状。因无法控制的瘘管或胃肠穿孔而停止使用CABOMETYX。•血栓形成事件:停止CABOMETYX用于心肌梗塞,脑梗塞或其他严重的血栓栓塞事件。•高血压和高血压危机:定期监测血压。用抗高血压治疗未充分控制的高血压中断。
停止CABOMETYX治疗无法控制的高血压危象或严重高血压抗高血压治疗。•腹泻:可能很严重。中断CABOMETYX立即降低腹泻,结果或降至等级。推荐标准的腹泻治疗。•手掌足底红斑感觉(PPE):中断CABOMETYX治疗直至PPE结算或降至等级。•蛋白尿:监测尿蛋白。停止肾病综合症。•下颌骨坏死:在进行侵入性牙科手术和开发ONJ之前,至少保留CABOMETYX至少28天。•伤口并发症:扣留CABOMETYX用于需要医疗干预的裂开或并发症。•可逆性后部白质脑病综合征(RPLS):停止使用CABOMETYX。•胚胎 - 胎儿毒性:可能导致胎儿伤害。建议女性有可能对胎儿造成潜在风险,并在治疗期间和最后一次给药后4个月内使用有效的感染。
不良反应
最常见(≥25%)的不良反应是:腹泻,疲劳,皮肤萎缩,手掌-足底红斑感觉(PPE),恶心,高血压和呕吐。
药物相互作用
•强CYP3A4抑制剂:如果无法避免给药,则减少CABOMETYX剂量。•强CYP3A4诱导剂:如果无法避免给药,则增加CABOMETYX剂量。
用于特定人群
•肝功能损害:减少中度肝功能损害患者的CABOMETYX剂量。避免患有严重肝病的患者损害。•哺乳期:建议不要母乳喂养。
包装提供/存储和处理
CABOMETYX片剂供货如下:60mg片剂是黄色薄膜包衣,椭圆形,没有刻痕,在一侧用“XL”压印,在另一侧用“60”压印; 有30片装:NDC 42388-023-2640mg片剂是黄色薄膜包衣,三角形没有刻痕,在一侧用“XL”压印,在另一侧用“40”压印; 有30片装:NDC 42388-025-2620mg片剂是黄色薄膜包衣,圆形没有刻痕,在一侧用“XL”压印,在另一侧用“20”压印; 有30片装:NDC 42388-024-26将CABOMETYX储存在20°C至25°C(68°F至77°F); 允许从15°C到30°C(59°F至86°F)[见USP受控室温]。
英文版说明书
Food and Drug Administration approved Cabometyx (cabozantinib) tablets for the treatment of patients with hepatocellular carcinoma (HCC) who have been previously treated with Nexavar (sorafenib).The Food and Drug Administration (FDA) approved Cabometyx (cabozantinib) tablets for the treatment of patients with hepatocellular carcinoma (HCC) who have been previously treated with Nexavar (sorafenib), according to Exelixis, the drug’s manufacturer.“This new indication for CABOMETYX is an important treatment advance for patients with this aggressive form of liver cancer, a community in need of new therapeutic options,” said Michael M. Morrissey, Ph.D., president and chief executive officer of Exelixis, said in a press release.The agency based its approval on results from the pivotal phase 3 CELESTIAL trial – designed to eva luate Cabometyx compared with placebo in 707 patients with advanced HCC, which is the most common form of liver cancer and the fastest-rising cause of cancer-related death in the U.S., according to the release.In the randomized, double-blind, placebo-controlled study, patients were randomized to receive 60 mg of Cabometyx daily (470 patients) or placebo (237 patients).Overall survival served as the primary endpoint, while secondary endpoints included objective response rate and progression-free survival – or the time until disease progression or worsening.Patients treated with Cabometyx demonstrated superior overall survival compared with placebo (10.2 vs. 8months), and median progression-free survival was more than double (5.2 vs. 1.9 months) compared with placebo.Four percent of percent of patients in the Cabometyx arm experienced an objective response rate compared with .04 percent of those who received placebo. In addition, disease control (partial response or stable disease) was achieved by 64 percent compared with 33 percent, respectively.The safety profile of Cabometyx appeared consistent with previous findings. The most common grade 3 or 4 side effects in the treatment arm included hand-foot syndrome (17 percent vs. 0 percent), hypertension (16 percent vs. 2 percent), increased aspartate aminotransferase (12 percent vs. 7 percent), fatigue (10 percent vs. 4 percent) and diarrhea (10 percent vs. 2 percent) compared with placebo. Sixteen percent of patients in the Cabometyx arm and three percent of patients in the placebo arm discontinued treatment due to treatment-related side effects.“Patients with this form of advanced liver cancer have few treatment options, particularly once their disease progresses following treatment with sorafenib,” lead investigator Ghassan K. Abou-Alfa, M.D., from Memorial Sloan Kettering Cancer Center, said in the release. “Physicians are eager for new options for these patients, and the results of the CELESTIAL trial demonstrate that Cabometyx has the efficacy and safety profile to become an important new therapy in our efforts to slow disease progression and improve treatment outcomes.”
用药温馨提示:当您服用此药物时,需定期接受医疗专业人士的检查,以便随时针对其药效、副作用等情况进行监测。本网站所包含的信息旨在为患者提供帮助,不能代替医学建议和治疗。
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 卡赞替尼片Cabometyx Tablets 60mg(cabozantinib)
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 卡赞替尼片Cabometyx Tablets 60mg(cabozantinib)


