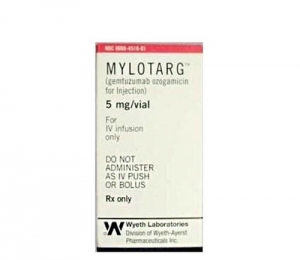
氯法拉滨注射溶液CLOLAR 1MG/ML 20ML SDV(CLOFARABINE)
 药店国别:
产地国家:美国
处方药:是
所属类别: 1毫克/毫升 20毫升/瓶
包装规格: 1毫克/毫升 20毫升/瓶
计价单位:瓶
生产厂家中文参考译名:
生产厂家英文名:SANOFI-AVENTIS
原产地英文商品名:CLOLAR 1MG/ML 20ML SDV PF DPSH 1/EA
原产地英文药品名:CLOFARABINE
中文参考商品译名:克罗拉注射溶液 1毫克/毫升 20毫升/瓶
中文参考药品译名:氯法拉滨
曾用名:
简介:
抗癌药Clolar(clofarabine)是用于治疗淋巴细胞白血病的抗肿瘤嘌呤核苷类似物。该药物作为抗代谢物,干扰DNA复制。此外,药物似乎破坏了线粒体膜的完整性,释放促凋亡线粒体蛋白,细胞色素C和细胞凋亡诱导因子,激活程序性细胞死亡的途径。以这些方式,Clolar对快速分裂和静止的癌细胞都产生细胞毒性作用。Clolar特异性表示治疗急性淋巴细胞性白血病(ALL)在1-21岁的儿科患者,其疾病在至少两个以前的治疗方案之后复发或复发性难治性。Clolar作为静脉内施用,推荐剂量为52mg/m2,通过2小时输液透析5天,一旦器官功能恢复到基线,每2-6周循环重复一次。批准日期:公司:赛诺菲 - 安万特Clolar(氯法拉滨 clofarabine)注射用于静脉注射美国最初批准:2004年最近的重大变化警告和注意事项:09/2014
作用机制
氯法拉滨通过脱氧胞苷激酶和单磷酸激酶和二磷酸激酶在细胞内依次代谢为5'-单磷酸代谢物,形成活性5'-三磷酸代谢物。Clofarabine对活化磷酸化酶,脱氧胞苷激酶具有亲和力,其等于或大于天然底物脱氧胞苷。氯法拉滨通过对核糖核苷酸还原酶的抑制作用减少细胞脱氧核苷酸三磷酸酶池,并通过竞争性抑制DNA聚合酶终止DNA链延伸并通过掺入DNA链来抑制修复,从而抑制DNA合成。亲和力这些酶的氯法拉滨三磷酸盐类似于或大于脱氧腺苷三磷酸盐。在临床前模型中,氯法拉滨已经证明了在修复过程中通过掺入DNA链来抑制DNA修复的能力。Clofarabine 5'triphosphate也破坏线粒体膜的完整性,导致促凋亡的线粒体蛋白,细胞色素C和凋亡诱导因子的释放,导致编程序列细胞死亡。Clofarabine对体外快速增殖和静止的癌细胞类型具有细胞毒性。
适应症和用法
Clolar(氯法拉滨)注射液是一种嘌呤核苷代谢抑制剂,用于治疗1至21岁患有复发或难治性急性淋巴细胞白血病的儿科患者,至少有两种治疗方案。该适应症基于反应率。没有试验验证疾病相关症状的改善或Clolar的存活率增加。
剂量和给药
在28天周期内连续5天,每天2小时静脉输注52mg/m2的推荐儿科剂量。每2-6周重复一次。在Clolar给药的5天内提供支持性护理,例如静脉输液,抗高尿酸治疗和尿碱化,以降低肿瘤溶解的风险等不良事件。如果在给药5天期间出现低血压,则停止使用Clolar。减少肾功能损害患者的剂量。使用剂量修改毒性。剂量形式和强度20毫克/20毫升一次性小瓶。
禁忌症
没有。
警告和注意事项
骨髓抑制:可能是严重和长期的。在Clolar治疗期间监测完整的血液计数和血小板计数。出血:严重致命的脑,胃肠和肺出血。监测血小板和凝血参数并相应地进行治疗。感染:由于骨髓抑制导致的严重和致命的败血症。监测感染的体征和症状;停止治疗并及时治疗。肿瘤裂解综合征:预期,监测体征和症状并及时治疗。全身炎症反应综合征(SIRS)或毛细血管综合征:如果怀疑,立即监测和停止Clolar。肝静脉闭塞性疾病:如果怀疑,监测并停止使用。肝毒性:严重致命的肝毒性。监测肝脏酶并停止Clolar。肾毒性:肌酐增加和急性肾功能衰竭; monitorrenal功能和中断或停止Clolar。小肠结肠炎:严重致命的小肠结肠炎,在治疗30天和联合化疗后更频繁发生。监测患者的小肠结肠炎症状和体征并及时治疗。皮肤反应:Stevens-Johnson综合征(SJS)和中毒性表皮溶解(TEN),包括致命病例。停止剥脱性皮疹,或怀疑是SJS或TEN。
不良反应
最常见的不良反应(10%):恶心,呕吐,腹泻,发热性胃痛,头痛,皮疹,瘙痒,发热,疲劳,手掌 - 植物性心律失常综合症,焦虑,潮红和粘膜炎症。
用于特定人群
成人尚未确定安全性和有效性。胚胎-胎儿毒性:当给予孕妇时,可能会发生胎儿伤害。应该建议女性在接受Clolar时避免成为怀孕者。
如何提供/存储和处理
Clolar(氯法拉滨)注射液在一次性燧石小瓶中提供,该小瓶含有20mg氯法拉滨20mL溶液。 每个盒子包含一个Clolar小瓶(NDC 0024-5860-01。)20mL flintvials含有20mL(20mg)溶液。溶液的pH范围为4.5至7.5。含有未稀释的Clolar的小瓶应储存在25°C(77°F); 短途旅行允许15至30°C(59 - 86°F)。稀释的外加剂可以在室温下储存,但必须在制备后24小时内使用。应使用适当处理和处置的程序。 Clolars的处理和处置应符合细胞毒性药物的指导原则。 关于这一主题的若干指导方针已经出版。
氯法拉滨注射溶液英文版说明书
UGNE8J4E/021673s023lbl.pdfClolar(clofarabine injection)PrefaceClolaris the brand name of the medication Clofarabine, a medicine used to treat cancer. Clolar is currently indicated to treat acute lymphoblastic leukemia (ALL) in pediatric patients aged 1 to 21 years old that has relapsed after at least two prior treatments.This is the only indication of Clolar, and it cannot be used for treatment of new ALL cases and for other types of cancer. Clolar is indicated for pediatric refractory ALL based on its response rate alone and there were no trials showing that it can extend survival or improve disease-related symptoms.Acute lymphoblastic leukemia is a type of blood and bone marrow cancer. ALL happens when too many stem cells from the bone marrow becomes lymphoblasts (hence, the term ‘lymphoblastic’), B lymphocytes and T lymphocytes. These cells are not normal, and not able to hunt and fight infectious pathogens well. Also, the sudden drastic increase of these cells create less room for red blood cells, white blood cells and platelets, reducing their number and causing hallmark symptoms like anaemia, frequent infections and bleeding tendencies.The huge numbers of useless, cancerous cells spreads in via bloodstream and lymph vessels to lymph nodes and other organs to cause complications.ALL is termed ‘acute’ because it progresses fairly quickly in short amount of time and can cause death in a few months. ALL is the most common form of blood cancer in children. When treated early, ALL treatment can be successful. If ALL recurs or relapses after one or two treatments, it is already considered a refractory case because it does not respond to usual medications. Only medicines like Clolar are found to cause good response to refractory cases of ALL.Clolar is manufactured and owned by Genzyme Corporation, a biotechnology company based in Cambridge, Massachusetts which is now a fully owned subsidiary of the French-owned Sanofi S.A. Clolar first entered the market after receiving approval from the United States Food and Drug Administration (FDA) on December 2004. Clolar is classified as an orphan medication and thus not widely available in most pharmacies, even in countries where it is approved.CANCER TREATMENT MEDS dispenses Clolar and delivers it to customers on a prescription-only basis. We are a premier internet pharmacy specializing on orphan medicines such as Clolar, and we deliver to most countries. We are your best source of Clolar; order it now at CANCER TREATMENT MEDS!There is no information indicating the use of Clolar for sarcomas. Clolar is exclusively indicated to treat pediatric cases of refractory ALL. There is no research study or clinical trials supporting the use of Clolar for sarcoma-type cancers or for soft-tissue sarcoma. There is also no documented off-label use of Clolar to treat sarcomas.Clolar medication and useClolar medication is used to treat ALL cases in persons below 21 years old that has recurred despite two previous treatments. Pediatric cases of ALL are usually treated with chemotherapy, radiotherapy or a combination of the two. In case of remission, high-dose treatment of chemotherapy with radiotherapy plus stem cell support is indicated to improve chances of cure or prolong remission. If despite second treatment there is still another remission of ALL, Clolar can now be considered to be used for treatment.Clolar is supplied in a single-use vial containing 20 mg of Clofarabine dissolved in 20 mL 0.9% sodium chloride injection, USP. The recommended dosage of Clolar is 52 mg/m2 of patient’s body surface area, which will be computed by your oncologist.To administer, Clolar must be first filtered through a sterile 0.2 micron syringe filter, then diluted to intravenous fluids 5% Dextrose Injection, USP, or 0.9% Sodium Chloride Injection, USP prior to infusion to achieve final concentration between 0.15 mg/mL and 0.4 mg/mL. Diluted Clolar must be used within 24 hours, and stored at room temperatures 15 C- 30 C. Your doctor or nurse will prepare Clolar for you. Clolar must be infused through an exclusive intravenous line; no other medicines must be infused in this line to prevent drug incompatibilities.Clolar infusions are given for over 2 hours for 5 consecutive days.Then a waiting period of 2 to 5 weeks is initiated until organ function tests results return to baseline, and Clolar is infused again. During Clolar infusion days, your kidney, heart and liver functions are closely monitored and supportive care such as intravenous fluids and antihyperuremic treatmentare provided. If your creatinine clearance is goes down to between 30 – 60 mg/minute, Clolar dosage is reduced by 50%.Furthermore, additional medications such as anti-emetics and prophylactic steroids are given during Clolar infusions.Clolar dosage may be adjusted if the following toxicities occur:•For grade 3 neutropenia lasting ≥ 4 weeks, Clolar dose is reduced by 25% for the next cycle•Clolar dose is withheld if the patient develops significant infection, which is then restarted when it subsides•Withhold Clolar dose in any event of Grade 3 non-infectious non-hematological toxicity, but not including momentary elevations in serum transaminase and/or bilirubin, and nausea and vomiting controlled by antiemetic therapy. When these toxicities subside, re-administer Clolar with 25% dose reduction•If there is any event of Grade 4 non-infectious non-hematological toxicity, discontinue Clolar administration•If systemic inflammatory response syndrome (SIRS) occur or capillary leak occurs, discontinue Clolar administration•If there is Grade 3 or higher creatinine and bilirubin increases, discontinue Clolar administration. If organ functions returns to baseline and becomes stable, re-administer Clolar at the next scheduled dose with a 25% dose reductionClolar chemical structureClolar has a chemical structure formula C10H11ClFN5O3 and molecular weight of 303.677 g/mol.Clolar, in its vial, appears as a clear and colourless liquid.Clolar in vials is sterile and preservative-free, and therefore can only be used once.According to a study involving 40 ALL patients aged 2 to 19 years of age, Clolar is 47% bound to plasma proteins and concentrations were seen over wide range of body surface areas. Clolar has a half-life of 2.5 hours in the body, and 49-60% of it is excreted in the urine unchanged. The pathways of Clolar to the hepatic (liver) system are not yet known.Clolar definitionClolar is the brand name of the medication Clofarabine. Clofarabine is a medication indicated to treat pediatric cases of recurrent or refractory ALL.Clolar classificationClolar is classified as an antineoplastic medication working as a purine nucleoside metabolic inhibitor indicated for treatment of ALL.
药店国别:
产地国家:美国
处方药:是
所属类别: 1毫克/毫升 20毫升/瓶
包装规格: 1毫克/毫升 20毫升/瓶
计价单位:瓶
生产厂家中文参考译名:
生产厂家英文名:SANOFI-AVENTIS
原产地英文商品名:CLOLAR 1MG/ML 20ML SDV PF DPSH 1/EA
原产地英文药品名:CLOFARABINE
中文参考商品译名:克罗拉注射溶液 1毫克/毫升 20毫升/瓶
中文参考药品译名:氯法拉滨
曾用名:
简介:
抗癌药Clolar(clofarabine)是用于治疗淋巴细胞白血病的抗肿瘤嘌呤核苷类似物。该药物作为抗代谢物,干扰DNA复制。此外,药物似乎破坏了线粒体膜的完整性,释放促凋亡线粒体蛋白,细胞色素C和细胞凋亡诱导因子,激活程序性细胞死亡的途径。以这些方式,Clolar对快速分裂和静止的癌细胞都产生细胞毒性作用。Clolar特异性表示治疗急性淋巴细胞性白血病(ALL)在1-21岁的儿科患者,其疾病在至少两个以前的治疗方案之后复发或复发性难治性。Clolar作为静脉内施用,推荐剂量为52mg/m2,通过2小时输液透析5天,一旦器官功能恢复到基线,每2-6周循环重复一次。批准日期:公司:赛诺菲 - 安万特Clolar(氯法拉滨 clofarabine)注射用于静脉注射美国最初批准:2004年最近的重大变化警告和注意事项:09/2014
作用机制
氯法拉滨通过脱氧胞苷激酶和单磷酸激酶和二磷酸激酶在细胞内依次代谢为5'-单磷酸代谢物,形成活性5'-三磷酸代谢物。Clofarabine对活化磷酸化酶,脱氧胞苷激酶具有亲和力,其等于或大于天然底物脱氧胞苷。氯法拉滨通过对核糖核苷酸还原酶的抑制作用减少细胞脱氧核苷酸三磷酸酶池,并通过竞争性抑制DNA聚合酶终止DNA链延伸并通过掺入DNA链来抑制修复,从而抑制DNA合成。亲和力这些酶的氯法拉滨三磷酸盐类似于或大于脱氧腺苷三磷酸盐。在临床前模型中,氯法拉滨已经证明了在修复过程中通过掺入DNA链来抑制DNA修复的能力。Clofarabine 5'triphosphate也破坏线粒体膜的完整性,导致促凋亡的线粒体蛋白,细胞色素C和凋亡诱导因子的释放,导致编程序列细胞死亡。Clofarabine对体外快速增殖和静止的癌细胞类型具有细胞毒性。
适应症和用法
Clolar(氯法拉滨)注射液是一种嘌呤核苷代谢抑制剂,用于治疗1至21岁患有复发或难治性急性淋巴细胞白血病的儿科患者,至少有两种治疗方案。该适应症基于反应率。没有试验验证疾病相关症状的改善或Clolar的存活率增加。
剂量和给药
在28天周期内连续5天,每天2小时静脉输注52mg/m2的推荐儿科剂量。每2-6周重复一次。在Clolar给药的5天内提供支持性护理,例如静脉输液,抗高尿酸治疗和尿碱化,以降低肿瘤溶解的风险等不良事件。如果在给药5天期间出现低血压,则停止使用Clolar。减少肾功能损害患者的剂量。使用剂量修改毒性。剂量形式和强度20毫克/20毫升一次性小瓶。
禁忌症
没有。
警告和注意事项
骨髓抑制:可能是严重和长期的。在Clolar治疗期间监测完整的血液计数和血小板计数。出血:严重致命的脑,胃肠和肺出血。监测血小板和凝血参数并相应地进行治疗。感染:由于骨髓抑制导致的严重和致命的败血症。监测感染的体征和症状;停止治疗并及时治疗。肿瘤裂解综合征:预期,监测体征和症状并及时治疗。全身炎症反应综合征(SIRS)或毛细血管综合征:如果怀疑,立即监测和停止Clolar。肝静脉闭塞性疾病:如果怀疑,监测并停止使用。肝毒性:严重致命的肝毒性。监测肝脏酶并停止Clolar。肾毒性:肌酐增加和急性肾功能衰竭; monitorrenal功能和中断或停止Clolar。小肠结肠炎:严重致命的小肠结肠炎,在治疗30天和联合化疗后更频繁发生。监测患者的小肠结肠炎症状和体征并及时治疗。皮肤反应:Stevens-Johnson综合征(SJS)和中毒性表皮溶解(TEN),包括致命病例。停止剥脱性皮疹,或怀疑是SJS或TEN。
不良反应
最常见的不良反应(10%):恶心,呕吐,腹泻,发热性胃痛,头痛,皮疹,瘙痒,发热,疲劳,手掌 - 植物性心律失常综合症,焦虑,潮红和粘膜炎症。
用于特定人群
成人尚未确定安全性和有效性。胚胎-胎儿毒性:当给予孕妇时,可能会发生胎儿伤害。应该建议女性在接受Clolar时避免成为怀孕者。
如何提供/存储和处理
Clolar(氯法拉滨)注射液在一次性燧石小瓶中提供,该小瓶含有20mg氯法拉滨20mL溶液。 每个盒子包含一个Clolar小瓶(NDC 0024-5860-01。)20mL flintvials含有20mL(20mg)溶液。溶液的pH范围为4.5至7.5。含有未稀释的Clolar的小瓶应储存在25°C(77°F); 短途旅行允许15至30°C(59 - 86°F)。稀释的外加剂可以在室温下储存,但必须在制备后24小时内使用。应使用适当处理和处置的程序。 Clolars的处理和处置应符合细胞毒性药物的指导原则。 关于这一主题的若干指导方针已经出版。
氯法拉滨注射溶液英文版说明书
UGNE8J4E/021673s023lbl.pdfClolar(clofarabine injection)PrefaceClolaris the brand name of the medication Clofarabine, a medicine used to treat cancer. Clolar is currently indicated to treat acute lymphoblastic leukemia (ALL) in pediatric patients aged 1 to 21 years old that has relapsed after at least two prior treatments.This is the only indication of Clolar, and it cannot be used for treatment of new ALL cases and for other types of cancer. Clolar is indicated for pediatric refractory ALL based on its response rate alone and there were no trials showing that it can extend survival or improve disease-related symptoms.Acute lymphoblastic leukemia is a type of blood and bone marrow cancer. ALL happens when too many stem cells from the bone marrow becomes lymphoblasts (hence, the term ‘lymphoblastic’), B lymphocytes and T lymphocytes. These cells are not normal, and not able to hunt and fight infectious pathogens well. Also, the sudden drastic increase of these cells create less room for red blood cells, white blood cells and platelets, reducing their number and causing hallmark symptoms like anaemia, frequent infections and bleeding tendencies.The huge numbers of useless, cancerous cells spreads in via bloodstream and lymph vessels to lymph nodes and other organs to cause complications.ALL is termed ‘acute’ because it progresses fairly quickly in short amount of time and can cause death in a few months. ALL is the most common form of blood cancer in children. When treated early, ALL treatment can be successful. If ALL recurs or relapses after one or two treatments, it is already considered a refractory case because it does not respond to usual medications. Only medicines like Clolar are found to cause good response to refractory cases of ALL.Clolar is manufactured and owned by Genzyme Corporation, a biotechnology company based in Cambridge, Massachusetts which is now a fully owned subsidiary of the French-owned Sanofi S.A. Clolar first entered the market after receiving approval from the United States Food and Drug Administration (FDA) on December 2004. Clolar is classified as an orphan medication and thus not widely available in most pharmacies, even in countries where it is approved.CANCER TREATMENT MEDS dispenses Clolar and delivers it to customers on a prescription-only basis. We are a premier internet pharmacy specializing on orphan medicines such as Clolar, and we deliver to most countries. We are your best source of Clolar; order it now at CANCER TREATMENT MEDS!There is no information indicating the use of Clolar for sarcomas. Clolar is exclusively indicated to treat pediatric cases of refractory ALL. There is no research study or clinical trials supporting the use of Clolar for sarcoma-type cancers or for soft-tissue sarcoma. There is also no documented off-label use of Clolar to treat sarcomas.Clolar medication and useClolar medication is used to treat ALL cases in persons below 21 years old that has recurred despite two previous treatments. Pediatric cases of ALL are usually treated with chemotherapy, radiotherapy or a combination of the two. In case of remission, high-dose treatment of chemotherapy with radiotherapy plus stem cell support is indicated to improve chances of cure or prolong remission. If despite second treatment there is still another remission of ALL, Clolar can now be considered to be used for treatment.Clolar is supplied in a single-use vial containing 20 mg of Clofarabine dissolved in 20 mL 0.9% sodium chloride injection, USP. The recommended dosage of Clolar is 52 mg/m2 of patient’s body surface area, which will be computed by your oncologist.To administer, Clolar must be first filtered through a sterile 0.2 micron syringe filter, then diluted to intravenous fluids 5% Dextrose Injection, USP, or 0.9% Sodium Chloride Injection, USP prior to infusion to achieve final concentration between 0.15 mg/mL and 0.4 mg/mL. Diluted Clolar must be used within 24 hours, and stored at room temperatures 15 C- 30 C. Your doctor or nurse will prepare Clolar for you. Clolar must be infused through an exclusive intravenous line; no other medicines must be infused in this line to prevent drug incompatibilities.Clolar infusions are given for over 2 hours for 5 consecutive days.Then a waiting period of 2 to 5 weeks is initiated until organ function tests results return to baseline, and Clolar is infused again. During Clolar infusion days, your kidney, heart and liver functions are closely monitored and supportive care such as intravenous fluids and antihyperuremic treatmentare provided. If your creatinine clearance is goes down to between 30 – 60 mg/minute, Clolar dosage is reduced by 50%.Furthermore, additional medications such as anti-emetics and prophylactic steroids are given during Clolar infusions.Clolar dosage may be adjusted if the following toxicities occur:•For grade 3 neutropenia lasting ≥ 4 weeks, Clolar dose is reduced by 25% for the next cycle•Clolar dose is withheld if the patient develops significant infection, which is then restarted when it subsides•Withhold Clolar dose in any event of Grade 3 non-infectious non-hematological toxicity, but not including momentary elevations in serum transaminase and/or bilirubin, and nausea and vomiting controlled by antiemetic therapy. When these toxicities subside, re-administer Clolar with 25% dose reduction•If there is any event of Grade 4 non-infectious non-hematological toxicity, discontinue Clolar administration•If systemic inflammatory response syndrome (SIRS) occur or capillary leak occurs, discontinue Clolar administration•If there is Grade 3 or higher creatinine and bilirubin increases, discontinue Clolar administration. If organ functions returns to baseline and becomes stable, re-administer Clolar at the next scheduled dose with a 25% dose reductionClolar chemical structureClolar has a chemical structure formula C10H11ClFN5O3 and molecular weight of 303.677 g/mol.Clolar, in its vial, appears as a clear and colourless liquid.Clolar in vials is sterile and preservative-free, and therefore can only be used once.According to a study involving 40 ALL patients aged 2 to 19 years of age, Clolar is 47% bound to plasma proteins and concentrations were seen over wide range of body surface areas. Clolar has a half-life of 2.5 hours in the body, and 49-60% of it is excreted in the urine unchanged. The pathways of Clolar to the hepatic (liver) system are not yet known.Clolar definitionClolar is the brand name of the medication Clofarabine. Clofarabine is a medication indicated to treat pediatric cases of recurrent or refractory ALL.Clolar classificationClolar is classified as an antineoplastic medication working as a purine nucleoside metabolic inhibitor indicated for treatment of ALL.
用药温馨提示:当您服用此药物时,需定期接受医疗专业人士的检查,以便随时针对其药效、副作用等情况进行监测。本网站所包含的信息旨在为患者提供帮助,不能代替医学建议和治疗。
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 氯法拉滨注射溶液CLOLAR 1MG/ML 20ML SDV(CLOFARABINE)
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 氯法拉滨注射溶液CLOLAR 1MG/ML 20ML SDV(CLOFARABINE)