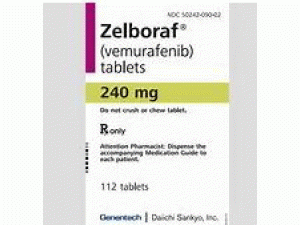
威罗菲尼片vemurafeni(Zelboraf 240mg Tablets)
 产地国家:日本
处方药:是
所属类别: 240毫克/片 56片/盒
包装规格: 240毫克/片 56片/盒
计价单位:盒
生产厂家英文名:Chugai
原产地英文商品名:Zelboraf(ゼルボラフ錠)240mg/tab 56tabs/box
原产地英文药品名:vemurafenib
中文参考商品译名:Zelboraf(ゼルボラフ錠)240毫克/片 56片/盒
中文参考药品译名:威罗菲尼
产地国家:日本
处方药:是
所属类别: 240毫克/片 56片/盒
包装规格: 240毫克/片 56片/盒
计价单位:盒
生产厂家英文名:Chugai
原产地英文商品名:Zelboraf(ゼルボラフ錠)240mg/tab 56tabs/box
原产地英文药品名:vemurafenib
中文参考商品译名:Zelboraf(ゼルボラフ錠)240毫克/片 56片/盒
中文参考药品译名:威罗菲尼
简介:
部份中文威罗菲尼处方资料(仅供参考)英文名:vemurafeni商标名:Zelboraf Tablets中文名:威罗菲尼生产商:罗氏(Roche)药物分类名称抗肿瘤药BRAF抑制剂商標名ZELBORAF一般名ベムラフェニブ(Vemurafenib)(JAN) 化学名:N-{3-[5-(4-Chlorophenyl)-1H-pyrrolo[2, 3-b]pyridin-3-carbonyl]-2, 4-difluorophenyl}propane-1-sulfonamide 分子式:C23H18ClF2N3O3S 分子量:489.92 性 状:它是白色粉末或块状粉末。它易溶于N,N-二甲基乙酰胺,几乎不溶于丙酮,极难溶于乙醇,几乎不溶于水。熔点约271℃批准条件1.制定药品风险管理计划并适当实施。2.由于日本的临床试验数量非常有限,通过对所有病例进行使用结果调查,直到制造和销售后累积一定数量的病例数据为止,除了掌握使用该药物的患者的背景信息外,还应尽快收集有关该药物安全性和有效性的数据,并采取必要措施正确使用该药物。 药用药理学:抗肿瘤作用Vemlafenib在体外对人恶性黑素瘤衍生的Colo 829和A 375细胞系具有增殖抑制活性,其具有BRAF V600E突变和具有BRAF V600 D突变的人恶性黑素瘤衍生的WM 2664细胞系。另外,在皮下移植人恶性黑素瘤细胞系(BRAF V600E突变)(LOX,Colo 829和A 375)的裸鼠中,显示了通过给予韦拉非尼的肿瘤生长抑制作用。 作用机制:通过抑制包括BRAF V600突变(V600E,V600D,V600R,V600K,V600G,V600M)在内的活化突变体的BRAF激酶活性,vemlafenib通过BRAF激活抑制MEK和ERK的磷酸化,抑制携带BRAF V600突变的肿瘤的生长。 适应症:伴有BRAF基因突变的根治性切除的恶性黑色素瘤 用法与用量:成人,每天口服960毫克,一次是Bemrafenib。临床结果1. <日语结果>具有BRAF V600突变的根治性不可切除的恶性黑素瘤患者的I / II期临床试验(JO 28178试验)17)BRAF V600突变8)11例根治性可切除的恶性黑素瘤患者 进行I / II期研究,其中每天一次给予960mg,每天两次空腹(给药前禁食2小时,给药后1小时)。 评价疗效的9名受试者的反应效率注9)为75.0%(95%置信区间:34.9-96.8)。注8)使用Kobas BRAF V600突变检测试剂盒进行检测,该试剂盒被批准用于制造和销售作为伴随诊断试剂。注9)通过RECIST(ver 1.1)指南判断(CR + PR)2. <外国人的结果>注8)基团不能切除带有打算送给恶性黑色素瘤患者的阶段III / IV级化疗历史而不BRAF突变V600与未处理BRAF突变V600 III疗效不能切除期临床试验(NO25026)此类靶向恶性黑色素瘤患者675案件阶段III / IV级进行比较给药组注释10),每天两次组和这种药物一次每日施用达卡巴嗪1000毫克/米960毫克@ 2,每3周第三阶段开放标签,随机对照试验(2010年12月30日数据截止)的结果如下所示。在OS的分析,这种药物给药相对于达卡巴嗪给药组0.37(95%置信区间:0.26-0.55)组的危险比,并且中央值通过Kaplan-Meier法,达卡巴嗪给药组7.75个月估计(95%置信间隔:6.28-10.28),这种药物给药组9.23个月(95%置信区间:8.05-和不可达),OS的统计学显著延长被确认(非分层数秩检验,p < 0.0001)。在PFS分析中,达卡巴嗪治疗组的危险比为0.26(95%置信区间:0.20-0.33),Kaplan-Meier法估计的中位数为1.61个月(95%) 确诊间隔:1.58-1.74),PFS的统计学显着延长得到证实(非分层Log-rank检验,p <1.58-1.74)和5.32个月(95%置信区间:4.86-6.57)0.0001)。注10)超出批准的剂量·剂量。 包装:片剂,240毫克,56片(PTP 8片×7)英文版说明书:
Zervolaf: a drug for gene mutation malignant melanomaOn December 26, 2014, the manufacture and sale of the anticancer drug Bemurafenib (trade name Zelborough tablet 240 mg) was approved. Indications are "radical resectable malignant melanoma with BRAF gene mutation", orally administered 960 mg twice a day.Melanoma is a tumor in which malignant nevus cells producing skin pigment are said to be the most malignant among skin cancers. As the site of occurrence, the plantar bottom is the most frequently occurring and it may occur on any skin such as trunk, face, nail. Although there are opinions that ultraviolet rays are involved due to extremely high incidence of Caucasians, the details have not been elucidated. Clinical symptoms are varied, black spots of pigment spots or tumors are observed, and it is classified into four disease types (endblister type, nodule type, superficial spreading type, malignant obsidian type) depending on the rate of progress and symptoms.In 2012, an estimated 230,000 people worldwide are diagnosed with malignant melanoma. Treatment at an early stage can be cured mostly, but it is said that the 5-year survival rate will be around 10% if progression to the stage IV where metastasis is recognized in skin and lymph nodes.In addition to surgical therapy such as excision surgery, radiotherapy using fast neutron rays and heavy particle beams, hyperthermia, immunotherapy, chemotherapy, etc. are attempted as the treatment method at the present time. In Japan, standard chemotherapy for progressive malignant melanoma was only monotherapy of anticancer drug dacarbazine, but human anti-human PD-1 monoclonal antibody nibolumab (2014 years old) with a novel mechanism of action Product name Opivo) became clinically used.However, treatment options for malignant melanoma are still limited, and the prognosis of malignant melanoma patients who can not be resected by surgery is extremely poor, so development and approval of new drugs have been desired.BRAF is a kinase protein that plays an important role in cell proliferation signaling. It is believed that BRAF (BRAF V600) in which about 50% of metastatic malignant melanoma and valine at amino acid sequence 600 is mutated is expressed and is constantly activated to promote cell proliferation.This approved Vemlafenib is a low molecular molecular targeted drug with a novel mechanism of action that selectively inhibits BRAF V600 kinase, suppresses the proliferation of cancer cells and exerts antineoplastic effects.In overseas Phase 3 clinical trial (NO 25026 test) for patients with radical unresectable 3/4-year malignant melanoma with BRAF V600 gene mutation without history of chemotherapy, overall survival and Significant prolongation of progression - free survival period was also observed, with a significant difference also in response rate. Phase 1/2 clinical trial (JO 28178 test) was conducted in patients with BRAF V600 gene mutation in Japan, and the effectiveness and safety in Japanese have been confirmed.Note that side effects are recognized in all cases in domestic clinical trials. Major side effects were joint pain / rash (90.9% each), musculoskeletal pain / alopecia (63.6% each), fatigue (54.5%), and so on. Sclerocellular carcinoma, malignant tumor (secondary carcinogenesis), anaphylaxis, hypersensitivity and the like are recognized as serious side effects from overseas clinical trials and the like.In order to identify patients who are to be treated with bevacizumab, companion diagnostic agents that detect BRAF V600 gene mutations have already been included and released on the market.用药温馨提示:当您服用此药物时,需定期接受医疗专业人士的检查,以便随时针对其药效、副作用等情况进行监测。本网站所包含的信息旨在为患者提供帮助,不能代替医学建议和治疗。
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 威罗菲尼片vemurafeni(Zelboraf 240mg Tablets)
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 威罗菲尼片vemurafeni(Zelboraf 240mg Tablets)