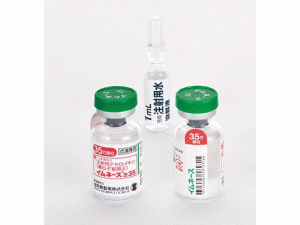
卡莫司汀植入片Gliadel 7.7mg Implan(Carmustine)
 药店国别:
产地国家:日本
处方药:是
所属类别: 7.7mg/枚 8枚/包
包装规格: 7.7mg/枚 8枚/包
计价单位:盒
生产厂家中文参考译名:
生产厂家英文名:Eisai Co. Ltd.
原产地英文商品名:Gliadel Implant 7.7mg/piece 8pieces/bag
原产地英文药品名:Carmustine
中文参考商品译名:Gliadel植入片(ギリアデル脳内留置用剤)7.7mg/枚 8枚/包(分包1枚×8)
中文参考药品译名:卡莫司汀
曾用名:
简介:部份中文卡莫司汀处方资料(仅供参考)
英文药名:Gliadel 7.7mgImplant(Carmustine)
中文药名:卡莫司汀植入片
药物分类名称:抗肿瘤药
批准日期:2012年11月
商標名:Gliadel 7.7mgImplant
一般名:カルムスチン(Carmustine)
化学名:1,3-Bis(2-chloroethyl)-1-nitrosourea
分子式:C5H9Cl2N3O2
分子量:214.05構造式性状它是一种淡黄色粉末。它非常易溶于二甲基亚砜,乙醇(95),二氯甲烷或乙腈,易溶于丙二醇,甲醇或无水二乙醚,不容易溶于水。
熔点:约31°C处理注意事项当卡莫司汀与皮肤接触时,可能会引起严重的灼伤和色素沉着,因此在处理本产品时要小心。
药效药理
1. 抗肿瘤效果已经证明卡莫司汀可以延长其移植有人成胶质细胞瘤的U-87MG细胞系的小鼠的存活时间。
2.作用机序据信卡莫司汀通过烷化DNA和抑制核酸合成来诱导细胞周期停滞和凋亡。
适应病症:恶性胶质瘤、脑瘤
用法与用量:通常,取决于肿瘤切除的腔的大小和形状,成年人在切除脑肿瘤时覆盖切除的表面,8片这种药物(61.6mg,作为卡莫司汀)或数量减少。
卡莫司汀英文版说明书
implanted antineoplastic drugs are listed in JapanNobelpharma and Eisai'simplantable antineoplastic agent 7.7 mg Gliadel (Caramustine) was listed on Jan.9 in Japan. Nobelpharma has completed a clinical study and obtainedmanufacturing and marketing rights in Japan on September 28, 2012 and wasregistered with the National Health Insurance (NHI) Drug List on 22 November2012. 7.7 mg Gliadel implants were promoted by cooperation between the twocompanies in Japan.Gliadel in Japan is the only approved slow-releasepreparation for intracranial implantation. Each wafer containsbischloroethylnitrosourea, an alkylation of nitrosourea, dispersed in abiodegradable polymer matrix. Malignant glioma resection after implantation ofthe wafer, can make chemotherapy drugs can be directly transmitted to the tumorsite.Gilladel's significant diagnosis of newly diagnosed malignant gliomassignificantly increased patient survival compared with placebo in a three-phasestudy conducted outside of Japan, whereas for patients with recurrentneuroblastoma after 6 months of total Survival rates also increased
significantly.Gliadel has now been approved in 30 countries around the world,including the United States, Europe and Southeast Asia.Glioma is a brain tumorthat accounts for 30% of all primary brain tumors. The incidence of malignantgliomas in Japan is estimated to be about 2000 to 2500 per year. Gliadel wasdesignated as a high demand drug by the Japanese Drug Unregistered UsageCommittee in September 2008 and was designated as orphan drug in June 2009
药店国别:
产地国家:日本
处方药:是
所属类别: 7.7mg/枚 8枚/包
包装规格: 7.7mg/枚 8枚/包
计价单位:盒
生产厂家中文参考译名:
生产厂家英文名:Eisai Co. Ltd.
原产地英文商品名:Gliadel Implant 7.7mg/piece 8pieces/bag
原产地英文药品名:Carmustine
中文参考商品译名:Gliadel植入片(ギリアデル脳内留置用剤)7.7mg/枚 8枚/包(分包1枚×8)
中文参考药品译名:卡莫司汀
曾用名:
简介:部份中文卡莫司汀处方资料(仅供参考)
英文药名:Gliadel 7.7mgImplant(Carmustine)
中文药名:卡莫司汀植入片
药物分类名称:抗肿瘤药
批准日期:2012年11月
商標名:Gliadel 7.7mgImplant
一般名:カルムスチン(Carmustine)
化学名:1,3-Bis(2-chloroethyl)-1-nitrosourea
分子式:C5H9Cl2N3O2
分子量:214.05構造式性状它是一种淡黄色粉末。它非常易溶于二甲基亚砜,乙醇(95),二氯甲烷或乙腈,易溶于丙二醇,甲醇或无水二乙醚,不容易溶于水。
熔点:约31°C处理注意事项当卡莫司汀与皮肤接触时,可能会引起严重的灼伤和色素沉着,因此在处理本产品时要小心。
药效药理
1. 抗肿瘤效果已经证明卡莫司汀可以延长其移植有人成胶质细胞瘤的U-87MG细胞系的小鼠的存活时间。
2.作用机序据信卡莫司汀通过烷化DNA和抑制核酸合成来诱导细胞周期停滞和凋亡。
适应病症:恶性胶质瘤、脑瘤
用法与用量:通常,取决于肿瘤切除的腔的大小和形状,成年人在切除脑肿瘤时覆盖切除的表面,8片这种药物(61.6mg,作为卡莫司汀)或数量减少。
卡莫司汀英文版说明书
implanted antineoplastic drugs are listed in JapanNobelpharma and Eisai'simplantable antineoplastic agent 7.7 mg Gliadel (Caramustine) was listed on Jan.9 in Japan. Nobelpharma has completed a clinical study and obtainedmanufacturing and marketing rights in Japan on September 28, 2012 and wasregistered with the National Health Insurance (NHI) Drug List on 22 November2012. 7.7 mg Gliadel implants were promoted by cooperation between the twocompanies in Japan.Gliadel in Japan is the only approved slow-releasepreparation for intracranial implantation. Each wafer containsbischloroethylnitrosourea, an alkylation of nitrosourea, dispersed in abiodegradable polymer matrix. Malignant glioma resection after implantation ofthe wafer, can make chemotherapy drugs can be directly transmitted to the tumorsite.Gilladel's significant diagnosis of newly diagnosed malignant gliomassignificantly increased patient survival compared with placebo in a three-phasestudy conducted outside of Japan, whereas for patients with recurrentneuroblastoma after 6 months of total Survival rates also increased
significantly.Gliadel has now been approved in 30 countries around the world,including the United States, Europe and Southeast Asia.Glioma is a brain tumorthat accounts for 30% of all primary brain tumors. The incidence of malignantgliomas in Japan is estimated to be about 2000 to 2500 per year. Gliadel wasdesignated as a high demand drug by the Japanese Drug Unregistered UsageCommittee in September 2008 and was designated as orphan drug in June 2009
用药温馨提示:当您服用此药物时,需定期接受医疗专业人士的检查,以便随时针对其药效、副作用等情况进行监测。本网站所包含的信息旨在为患者提供帮助,不能代替医学建议和治疗。
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 卡莫司汀植入片Gliadel 7.7mg Implan(Carmustine)
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 卡莫司汀植入片Gliadel 7.7mg Implan(Carmustine)