硼替佐米冻干用粉注射剂Bortezomib(velcade 3.5mg Pulver Inj)
[caption id="attachment_20083" align="alignleft" width="300"]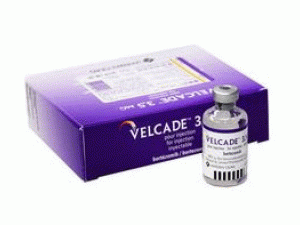 硼替佐米冻干用粉注射剂Bortezomib(velcade 3.5mg Pulver Inj)[/caption]
产地国家:德国
处方药:是
所属类别: 3.5毫克/瓶
包装规格: 3.5毫克/瓶
计价单位:瓶
生产厂家英文名:Janssen-Cilag GmbH
原产地英文商品名:VELCADE 3.5mg Pulver 1Stk
原产地英文药品名:Bortezomib
中文参考商品译名:Velcade冻干粉注射剂 3.5毫克/瓶
中文参考药品译名:硼替佐米
硼替佐米冻干用粉注射剂Bortezomib(velcade 3.5mg Pulver Inj)[/caption]
产地国家:德国
处方药:是
所属类别: 3.5毫克/瓶
包装规格: 3.5毫克/瓶
计价单位:瓶
生产厂家英文名:Janssen-Cilag GmbH
原产地英文商品名:VELCADE 3.5mg Pulver 1Stk
原产地英文药品名:Bortezomib
中文参考商品译名:Velcade冻干粉注射剂 3.5毫克/瓶
中文参考药品译名:硼替佐米
 硼替佐米冻干用粉注射剂Bortezomib(velcade 3.5mg Pulver Inj)[/caption]
产地国家:德国
处方药:是
所属类别: 3.5毫克/瓶
包装规格: 3.5毫克/瓶
计价单位:瓶
生产厂家英文名:Janssen-Cilag GmbH
原产地英文商品名:VELCADE 3.5mg Pulver 1Stk
原产地英文药品名:Bortezomib
中文参考商品译名:Velcade冻干粉注射剂 3.5毫克/瓶
中文参考药品译名:硼替佐米
硼替佐米冻干用粉注射剂Bortezomib(velcade 3.5mg Pulver Inj)[/caption]
产地国家:德国
处方药:是
所属类别: 3.5毫克/瓶
包装规格: 3.5毫克/瓶
计价单位:瓶
生产厂家英文名:Janssen-Cilag GmbH
原产地英文商品名:VELCADE 3.5mg Pulver 1Stk
原产地英文药品名:Bortezomib
中文参考商品译名:Velcade冻干粉注射剂 3.5毫克/瓶
中文参考药品译名:硼替佐米
简介:
部份中文万珂处方资料(仅供参考)英文药名: Velcade(Bortezomib Injection)中文药名: 万珂(硼替佐米注射液)生产厂家: Not available药品介绍通用名:注射用硼替佐米商品名:万珂TM(Velcade) 适应症:本品用于多发性骨髓瘤患者的治疗,此患者在使用本品前至少接受过两种治疗,并在最近-次治疗中病情还在进展。本品的有效性基于它的有效率。尚无临床对照试验证明其临床利益,如对存活率的改善 用法用量:本品的推荐剂量为单次注射1.3mg/㎡,每周注射2次,连续注射2周(即在第1、4、日和11天注射)后停药10天(即从第12至第2l天)。3周为1个疗程,两次给药至少间隔72小时。在临床研究中,被确认完全有效的患者再接受另外2个周期的注射用硼替佐米治疗。建议有效的患者接受8个周期的注射用硼替佐米治疗。 剂量调整以及重新开始治疗:当发生3级非血液学的或任何4级血液学的毒性(不包括下面讨论的神经病)时,应暂停本品治疗。一旦毒性症状得到缓解,可以重新开始本品的治疗,剂量减少25%(例如:1.3mg/㎡降低到1.0mg/㎡;1.0mg/㎡降低到0.7mg/㎡)。如果患者发生与本品治疗有关的神经痛或周围感觉神经病,应按下表推荐的调整剂量进行治疗。如果患者本身患有严重的神经病,只有权衡利弊后方可使用本品。 Velcade(Bortezomib Injection)为为全球第一个靶向型抑制肿瘤细胞的蛋白酶体药物硼替佐米作为全球第一个靶向型抑制肿瘤细胞的蛋白酶体药物,已被87个国家用于多发性骨髓瘤的治疗,并于2008年6月被美国FDA批准作为多发性骨髓瘤的一线治疗用药。2008年由美国、德国、英国的35家中心联合开展的Ⅱ期临床试验显示,硼替佐米单药治疗复发或难治性MCL,起效快(中位第一次缓解时间为2个疗程),缓解时间长(中位患者可延长13.5个月的生存期),对难治性和既往2次化疗无效的患者也有效。因此,FDA批准它作为MCL治疗的二线药物。目前我国也批准其用于MCL的单药治疗。 套细胞淋巴瘤是一组原发于淋巴结或结外部位淋巴组织的恶性肿瘤。在我国,MCL发病率占恶性肿瘤的第9位(男性)和第10位(女性),由于MCL较其他恶性肿瘤更具侵袭性,所以治疗的预后较差,是患者生存期最短的淋巴瘤亚型之一,以往没有有效的针对性药物 贮藏:在25℃(15-30℃)避光处保存。 规格:粉针 3.5mg、1.0mg英文版说明书:
VELCADE 1 mg powder for solution for injectionPublic Summary DocumentProduct: Bortezomib, powder for injection 1 mg (solvent required), Velcade®Sponsor: Janssen-Cilag Pty LtdDate of PBAC Consideration: March 20121. Purpose of ApplicationThe submission requested an extension to the current Authority Required listing to include induction therapy in a patient with newly diagnosed symptomatic multiple myeloma (MM) who is eligible for high dose chemotherapy, as part of combination therapy.From 1 December 2011, bortezomib has been included in the Revised Arrangements for the Efficient Funding of Chemotherapy and listed under Section 100.2. BackgroundBortezomib has not previously been considered by the PBAC for this indication.Bortezomib is currently PBS listed as a Section 100 (Efficient Funding of Chemotherapy) Public Hospital and Private Hospital/Private Clinic Authority Required benefit for the following indications:•treatment, as monotherapy or in combination with a corticosteroid and/or cyclophosphamide, of a patient with a histological diagnosis of multiple myeloma who has progressive disease after at least 1 prior therapy and who has undergone or is ineligible for a primary stem cell transplant;•treatment, as monotherapy or in combination with a corticosteroid and/or cyclophosphamide, of a patient with multiple myeloma who has progressive disease and who has been previously treated with PBS-subsidised bortezomib;PBS listing of bortezomib for treatment of patients with newly diagnosed symptomatic multiple myeloma who are ineligible for high dose chemotherapy, in combination with a corticosteroid and melphalan or cyclophosphamide is expected to proceed later in 2012.3. Registration StatusBortezomib 1 mg powder for injection was TGA registered on 1 June 2009 for the indication: as part of combination therapy, for induction therapy prior to high dose chemotherapy with autologous stem cell rescue for patients under 65 years of age with previously untreated multiple myeloma.Bortezomib is also TGA registered for the following indications:•in combination with melphalan and prednisone is indicated for the treatment of patients with previously untreated multiple myeloma who are not candidates for high dose chemotherapy.•for the treatment of multiple myeloma patients who have received at least one prior therapy, and who have progressive disease.4. Listing Requested and PBAC’s ViewNotePBS subsidised bortezomib will not be approved in combination with thalidomide or lenalidomide. No applications for increased maximum quantities and/or repeats will be authorised.Authority RequiredInitial PBS subsidised treatment, as part of combination therapy for induction therapy in a newly diagnosed patient with symptomatic multiple myeloma who is eligible for high dose chemotherapy.For PBAC’s view, see Recommendation and Reasons.5. Clinical Place for the Proposed TherapyMultiple myeloma is a cancer of plasma cells. It is a progressive haematological disease, which is incurable. Common clinical manifestations include hypercalcaemia, anaemia, renal damage, increased susceptibility to bacterial infection and impaired production of normal immunoglobulin. Diffuse osteoporosis, usually in the pelvis, spine, ribs and skull is also usually characteristic of MM.The submission proposed that the place in therapy of bortezomib, in combination therapy, is as an alternative to thalidomide and older induction chemotherapy regimens, such as VAD, prior to autologous stem cell transplant in patients with MM.6. ComparatorThe submission nominated thalidomide as the main comparator. The PBAC agreed that this was the appropriate comparator.7. Clinical TrialsThe basis of the submission was four randomised trials:•Two trials comparing bortezomib-based induction regimens with vincristine-doxorubicin-dexamethasone (VAD) (HOVON-65/GMMG-HD4 and IFM 2005-01); and•Two trials comparing thalidomide-based induction regimens with VAD (HOVON-50/GMMG-HD3 and Macro 2006.https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=1521D321-E724-4FFC-ADAD-34BF4F44FAC7用药温馨提示:当您服用此药物时,需定期接受医疗专业人士的检查,以便随时针对其药效、副作用等情况进行监测。本网站所包含的信息旨在为患者提供帮助,不能代替医学建议和治疗。
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 硼替佐米冻干用粉注射剂Bortezomib(velcade 3.5mg Pulver Inj)
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 硼替佐米冻干用粉注射剂Bortezomib(velcade 3.5mg Pulver Inj)