伊沙佐米胶囊ixazomib citrate(Ninlaro 4mg capsules 1X3ud)
 产地国家:美国
处方药:是
所属类别:4毫克/粒 1板X3粒
包装规格:4毫克/粒 1板X3粒
计价单位:盒
生产厂家英文名:NINLARO DIRECT/ICS
原产地英文商品名:NINLARO 4MG CAP 1X3UD DS
原产地英文药品名:IXAZOMIB CITRATE
中文参考商品译名:NINLARO胶囊 4毫克/粒 1板X3粒
中文参考药品译名:伊沙佐米
产地国家:美国
处方药:是
所属类别:4毫克/粒 1板X3粒
包装规格:4毫克/粒 1板X3粒
计价单位:盒
生产厂家英文名:NINLARO DIRECT/ICS
原产地英文商品名:NINLARO 4MG CAP 1X3UD DS
原产地英文药品名:IXAZOMIB CITRATE
中文参考商品译名:NINLARO胶囊 4毫克/粒 1板X3粒
中文参考药品译名:伊沙佐米
简介
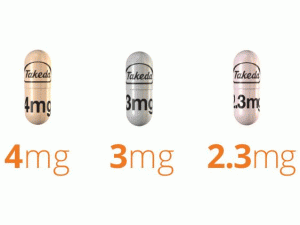
部份中文伊沙佐米处方资料(仅供参考) 英文名:Ixazomib 商品名:NINLARO Kaps 中文名:伊沙佐米胶囊 生产商:武田制药 药品介绍 近日,欧盟委员会(EC)已有条件批准口服蛋白酶体抑制剂Ninlaro(ixazomib),联合Revlimid(lenalidomide,来那度胺)及地塞米松(dexamethasone),用于既往已接受过至少一种治疗方案的多发性骨髓瘤(MM)成人患者。此次批准,使Ninlaro成为欧洲首个也是唯一一个口服蛋白酶体抑制剂。 作用机制 Ixazomib是一种可逆性蛋白体抑制剂。Ixazomib优先结合和抑制胰凝乳蛋白酶-样20S蛋白酶体的β5亚单位的活性。Ixazomib在体外诱导多发性骨髓瘤细胞系的凋亡。Ixazomib对来自多种以前治疗后,包括硼替佐米[bortezomib],来那度胺,和地塞米松已复发患者的骨髓瘤细胞显示体外细胞毒性。在多发性骨髓瘤细胞系中Ixazomib和来那度胺的联用显示协同的细胞毒效应。在体内,在一种小鼠多发性骨髓瘤肿瘤异种移植模型ixazomib显示抗肿瘤活性。 适应证:NINLARO是一个蛋白体抑制剂适用与来那度胺和地塞米松联用为有多发性骨髓瘤患者曽接受至少一种以前治疗的治疗。 剂量和给药方法:⑴ 推荐起始剂量4mg口服在28-天疗程的第1,8,和15天。⑵ 剂量应被服用食物前至少一小时或后至少2小时。 剂型和规格:胶囊:4mg,3mg,和2.3mg 禁忌症:无。 警告和注意事项 ⑴ 血小板减少:治疗期间监视血小板计数至少每月和调整,当需要时。 ⑵ 胃肠道毒性:对严重腹泻,便秘,恶心,和呕吐,当需要时调整给药。 ⑶ 外周神经病变:监视患者外周神经病变的症状和调整给药,当需要时。 ⑷ 外周水肿:监视液体潴留。研究潜在原因,当适当。调整给药,当需要时。 ⑸ 皮肤反应:监视患者皮疹和调整给药,当需要时。 ⑹ 肝毒性:治疗期间监视肝酶。 ⑺ 胚胎胎儿毒性:NINLARO可能致胎儿危害。忠告生殖潜能女性和使用有效避孕。 不良反应:最常见不良反应(≥ 20%)是腹泻,便秘,血小板减少,外周神经病变,恶心,外周水肿,呕吐,和背痛。 药物相互作用:强CYP3A诱导剂:避免与NINLARO同时使用。 特殊人群中使用:⑴ 肝受损:在有中度或严重肝受损患者减低NINLARO开始剂量至3mg。⑵ 肾受损:有严重肾受损或肾病终末期需要透析患者减低NINLARO开始剂量至3 mg。⑶ 哺乳:终止哺乳。英文版说明书
NINLARO(ixazomib) is the first and only oral medication of its kind, a proteasome inhibitor, that you can take at home. A study showed that adding NINLARO to REVLIMID(lenalidomide) and dexamethasone improved median progression-free survival (PFS) by nearly 6 months (20.6 months with the NINLARO regimen and 14.7 months with the placebo regimen).INDICATION AND IMPORTANT SAFETY INFORMATION FOR NINLARO(ixazomib)Uses of NINLARONINLARO is a prescription medicine used to treat multiple myeloma in combination with the medicines REVLIMID(lenalidomide) and dexamethasone, in people who have received at least one prior treatment for their multiple myeloma.It is not known if NINLARO is safe and effective in children.NINLARO may cause serious side effects, including:Low platelet counts (thrombocytopenia) are common with NINLARO and can sometimes be serious. You may need platelet transfusions if your counts are too low. Tell your healthcare provider if you have any signs of low platelet counts, including bleeding and easy bruising.Stomach and intestinal (gastrointestinal) problems. Diarrhea, constipation, nausea, and vomiting are common with NINLARO and can sometimes be severe. Call your healthcare provider if you get any of these symptoms and they do not go away during treatment with NINLARO. Your healthcare provider may prescribe medicine to help treat your symptoms.Nerve problems are common with NINLARO and may also be severe. Tell your healthcare provider if you get any new or worsening symptoms including: tingling, numbness, pain, a burning feeling in your feet or hands, or weakness in your arms or legs.Swelling is common with NINLARO and can sometimes be severe. Tell your healthcare provider if you develop swelling in your arms, hands, legs, ankles, or feet, or if you gain weight from swelling.Skin Reactions. Tell your healthcare provider if you get a new or worsening rash.Liver problems. Tell your healthcare provider if you get these signs of a liver problem: yellowing of your skin or the whites of your eyes; pain in your right upper-stomach area.Other common side effects have occurred. Tell your healthcare provider if you get new or worsening back pain, lowered white blood cells (neutropenia) that may increase the risk of infection, or vision conditions such as blurred vision, dry eye, or pink eye (conjunctivitis).These are not all the possible side effects of NINLARO. Talk to your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.Before taking NINLARO, tell your healthcare provider about all your medical conditions, including if:You have liver problems or kidney problems or are on dialysis.You or your partner are pregnant or plan to become pregnant. NINLARO can harm your unborn baby. Avoid becoming pregnant during treatment with NINLARO. You and your partner should use effective birth control during treatment and for 90 days after the final dose of NINLARO. If using hormonal contraceptives (for example, the pill), an additional barrier method of contraception (for example, diaphragm or condom) must be used.You are breastfeeding or plan to breastfeed. Do not breastfeed during treatment with NINLARO and for 90 days after your final dose of NINLARO.用药温馨提示:当您服用此药物时,需定期接受医疗专业人士的检查,以便随时针对其药效、副作用等情况进行监测。本网站所包含的信息旨在为患者提供帮助,不能代替医学建议和治疗。
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 伊沙佐米胶囊ixazomib citrate(Ninlaro 4mg capsules 1X3ud)
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 伊沙佐米胶囊ixazomib citrate(Ninlaro 4mg capsules 1X3ud)