端三环乙基Telotristat Ethyl(Xermelo Tablets 250mg)说明书
 产地国家:美国
处方药:是
所属类别: 250毫克/片 84片/瓶
包装规格: 250毫克/片 84片/瓶
计价单位:瓶
生产厂家英文名:Lexicon Pharmaceuticals,Inc.
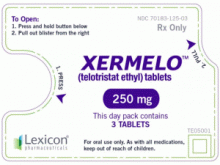
原产地英文商品名:Xermelo 250MG/Tablets 84Tablets
原产地英文药品名:telotristat ethyl
中文参考商品译名:Xermelo胶片 250毫克/片 84片/盒
中文参考药品译名:端三环乙基
产地国家:美国
处方药:是
所属类别: 250毫克/片 84片/瓶
包装规格: 250毫克/片 84片/瓶
计价单位:瓶
生产厂家英文名:Lexicon Pharmaceuticals,Inc.
原产地英文商品名:Xermelo 250MG/Tablets 84Tablets
原产地英文药品名:telotristat ethyl
中文参考商品译名:Xermelo胶片 250毫克/片 84片/盒
中文参考药品译名:端三环乙基
简介:
类癌综合征腹泻(CSD)的首个口服治疗药物Xermelo(telotristat ethyl,250mg) 片剂美国FDA批准近日,美国FDA批准Xermelo(telotristat ethyl,250mg) 片剂联合生长抑素类似物(somatostatin analog,SSA)疗法,用于单独接受SSA疗法无法充分控制病情的类癌综合征腹泻(carcinoid syndrome diarrhea,CSD)成人患者的治疗。此次批准,使Xermelo成为首个也是唯一一个获批治疗CSD的口服治疗药物,将为罹患类癌综合征腹泻的患者群体提供一种新的治疗选择。类癌综合征(Carcinoid syndrome)是一个集群的症状,有时可见于类癌肿瘤患者。类癌是一种罕见的、生长缓慢的、能产生小分子多肽类或肽类激素的肿瘤,是胃肠道最常见的内分泌肿瘤。类癌综合征在类癌肿瘤患者群体中的发生率低于10%,通常在肿瘤发生肝脏转移后出现。在这些患者中,肿瘤会释放过量的激素血清素,导致腹泻。而不受控的腹泻所伴随的并发症包括体重减轻、营养不良、脱水,以及电解质失衡。Xermelo由美国Lexicon制药公司开发,该药每日3次与食物同服。Xermelo 靶向类癌肿瘤细胞内的色氨酸羟化酶,通过抑制血清素(serotonin)的过量生产,降低类癌综合征腹泻的频率。批准日期:2017年2月28日;公司:Lexicon Pharmaceuticals,Inc.XERMELO(telotristat ethyl)片,为口服使用。
作用机制:Telotristat,telotristat ethyl的活性代谢物,是色氨酸羟化酶[tryptophan hydroxylase]的一种抑制剂,它介导在5-羟色胺生物合成中速率限制步骤。在体外telotristat对色氨酸羟化酶的抑制效力是较高于telotristat ethyl为29倍。5-羟色胺在介导胃肠道的分泌,运动,炎症,和感觉中起作用,而在有类癌综合证患者中过度生产。通过色氨酸羟化酶的抑制作用,telotristat和telotristat ethyl减低周围5-羟色胺的产生,和类癌综合证腹泻的频数。
适应证和用途:Xermelo是一种色氨酸羟化酶抑制剂适用为被SSA治疗控制不足成年用生长激素抑制素[somatostatin]类似物(SSA)治疗联用在类癌综合证腹泻的治疗。
剂量和给药方法:成年患者对腹泻被SSA治疗是控制不足患者Xermelo的推荐剂量是250 mg每天三次。与食物服用Xermelo。当短作用奥曲肽[octreotide]与Xermelo联用时,给予Xermelo后至少30分钟给予短作用奥曲肽。如发生严重便秘终止Xermelo.
剂型和规格:片剂,250mg telotristat ethyl
禁忌证:无
警告和注意事项:便秘:Xermelo减少肠运动频数;监视患者对便秘和/或严重持续或恶化的腹痛。如发生严重便秘或腹痛终止Xermelo。
不良反应:最常见不良反应(≥5%)为恶心,头痛,增加GGT,抑郁,胀气,食欲减退,周围水肿,和发热。
药物相互作用:CYP3A4底物(如,米达唑仑[midazolam]):同时药物的疗效可能被减低;监视对次优疗效和考虑增加同时药物的剂量。
贮存:25ºC(77ºF);外出允许至15ºC至30ºC(59ºF至86ºF)[见USP控制室温]。
英文版说明书:
Xermelo (telotristat ethyl)General InformationXermelo (telotristat ethyl) is a tryptophan hydroxylase inhibitor. Xermelo targets the overproduction of serotonin inside mNET cells.Xermelo is specifically indicated for the treatment of carcinoid syndrome diarrhea in combination with somatostatin analog (SSA) therapy in adults inadequately controlled by SSA therapy.Xermelo is supplied as a tablet for oral administration. The recommended dosage of Xermelo in adults is 250 mg three times daily for patients whose diarrhea is inadequately controlled by a SSA therapy. Xermelo should be ingested with food. When short-acting octreotide is used in combination with Xermelo, administer short-acting octreotide at least 30 minutes after administering Xermelo. Discontinue Xermelo if severe constipation develops.Clinical ResultsFDA ApprovalThe FDA approval of Xermelo was based on a 12-week double-blind, placebo-controlled, randomized, multicenter trial conducted in adult patients with a well-differentiated metastatic neuroendocrine tumor and carcinoid syndrome diarrhea who were having between 4 to 12 daily bowel movements despite the use of SSA therapy at a stable dose for at least 3 months. Patients were randomized to placebo or treatment with Xermelo 250 mg three times daily. Study medication was administered within 15 minutes before or within 1 hour after a meal or snack. All patients were required to stay on their baseline SSA regimen and were allowed to use rescue medication (short-acting octreotide) and antidiarrheals for symptomatic relief. A total of 90 patients were eva luated for efficacy. The primary efficacy endpoint was the change from baseline in the number of daily bowel movements averaged over the 12-week treatment period. In the 12-week study, a difference in average weekly reductions in bowel movement frequency between Xermelo and placebo was statistically significant and was observed as early as 1 to 3 weeks, and persisted for the remaining 9 weeks of the study.Mechanism of ActionXermelo (telotristat ethyl) is a tryptophan hydroxylase inhibitor. Tryptophan hydroxylase mediates the rate limiting step in serotonin biosynthesis. The in vitro inhibitory potency of telotristat towards tryptophan hydroxylase is 29 times higher than that of telotristat ethyl. Serotonin plays a role in mediating secretion, motility, inflammation, and sensation of the gastrointestinal tract, and is over-produced in patients with carcinoid syndrome. Through inhibition of tryptophan hydroxylase, telotristat and telotristat ethyl reduce the production of peripheral serotonin, and the frequency of carcinoid syndrome diarrhea.
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 端三环乙基Telotristat Ethyl(Xermelo Tablets 250mg)说明书