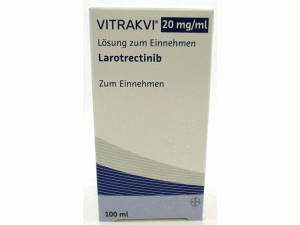
拉罗替尼口服溶液larotrectinib(Vitrakvi Oral Solution 20mg/mL)说明书
 产地国家:美国
处方药:是
所属类别: 200毫克/毫升/瓶
包装规格: 200毫克/毫升/瓶
计价单位:瓶
生产厂家英文名:BayerHealthCare Pharmaceuticals Inc
原产地英文商品名:Vitrakvi Oral Solution 20mg/mL/bottle
原产地英文药品名:larotrectinib
中文参考商品译名:Vitrakvi口服溶液 200毫克/毫升/瓶
中文参考药品译名:拉罗替尼
产地国家:美国
处方药:是
所属类别: 200毫克/毫升/瓶
包装规格: 200毫克/毫升/瓶
计价单位:瓶
生产厂家英文名:BayerHealthCare Pharmaceuticals Inc
原产地英文商品名:Vitrakvi Oral Solution 20mg/mL/bottle
原产地英文药品名:larotrectinib
中文参考商品译名:Vitrakvi口服溶液 200毫克/毫升/瓶
中文参考药品译名:拉罗替尼
简介:
近日,美国食品和药物管理局(FDA)加速批准Vitrakvi(larotrectinib)上市。用于治疗成人及小儿实体肿瘤患者有神经营养受体酪氨酸激酶(NTRK)基因融合没有已知获得性耐药突变,转移或手术切除可能导致严重的发病率,并无满意的替代治疗或后续治疗进展。根据总体响应速率(ORR)和响应持续时间(DOR),在加速审批下批准该指示。持续批准该适应症可能取决于验证性试验中的临床效益的验证和描述。Vitrakvi是第一个在FDA最初批准时获得肿瘤不可知适应症的治疗。批准日期:2018年11月26日 公司:BayerHealthCare Pharmaceuticals IncVITRAKVI(拉罗替尼[larotrectinib])胶囊,供口服使用VITRAKVI(拉罗替尼[larotrectinib])溶液,供口服使用美国初步批准日期:2018年 作用机制:Larotrectinib是原肌凝蛋白受体激酶(TRK)、TRKA、TRKB和trkc的抑制剂。在广泛的纯化酶分析中,larotrectinib抑制TRKA、TRKB和trkc, IC50值在5-11 nM之间。另一种激酶TNK2被抑制在大约100倍的高浓度。TRKA、B和C由genesNTRK1、NTRK2和NTRK3编码。染色体重排涉及这些基因与不同伴侣的框内融合,可导致本构激活嵌合TRK融合蛋白.这可以作为一个致癌的驱动因素,促进细胞增殖和肿瘤细胞系的生存。在体外和体内肿瘤模型中,larotrectinib在TRK蛋白本构激活的细胞中表现出抗肿瘤活性,这些细胞由基因融合、蛋白调控区域的缺失或TRK蛋白过表达引起。Larotrectinib在TRKA激酶域点突变的细胞系中活性最低,包括临床鉴定的获得性抗性突变G595R。TRKC激酶域的点突变包括G623R、G696A和F617L,临床上已确认对larotrectinib产生耐药。 适应症和用法:VITRAKVI是一种激酶抑制剂,适用于成人和儿童实体瘤患者的治疗:具有神经营养受体酪氨酸激酶(NTRK)基因融合,无aknown获得性耐药突变,是否转移或手术切除可能导致严重出血率,以及;没有令人满意的替代治疗或后续治疗有进展。该指示是在基于总体响应率和响应持续时间的加速审批下批准的。持续批准该指标可能取决于临床疗效验证和验证性试验的描述。 剂量和管理:根据aNTRK基因融合的存在,选择患者进行VITRAKVI治疗。成人和儿童体表表面积大于1.0米方的患者推荐剂量:每日口服两次,每次100毫克。儿童体表面积小于1.0米方的患者推荐剂量:每日2次口服100mg/m2。 剂型和强度:胶囊:25mg、100mg;口服:20mg/mL 禁忌症:没有。 警告和预防措施: 1.神经毒性:建议患者和护理人员注意神经系统不良反应的风险。建议有神经毒性的病人不要驾驶或操作危险的机器。保留和修改剂量,或根据严重程度永久停用VITRAKVI。 2.肝毒性:在治疗的第一个月,每2周监测一次包括ALT和AST在内的肝脏测试,然后按临床指示每月监测一次。保留和修改剂量,或根据严重程度永久停用VITRAKVI。 3.胚胎-胎儿毒性:可引起胎儿损害。建议对胎儿有潜在生殖风险的女性使用有效的镇痛药。 不良反应:最常见的不良反应(≥20%)和VITRAKVI疲劳、恶心、眩晕、呕吐、增加AST,咳嗽、ALT增加,便秘和腹泻。 药物的相互作用:1.强CYP3A4抑制剂:避免与VITRAKVI同时使用强CYP3A4inhibitors。如果不能避免联用,应减少VITRAKVI剂量。2.强CYP3A4诱导剂:避免与VITRAKVI同时使用强CYP3A4诱导剂。如果不能避免联用,增加VITRAKVI剂量。3.敏感CYP3A4底物:避免敏化yp3a4底物与VITRAKVI共服。 在特定人群中使用:哺乳期:建议不要母乳喂养。肝损害:中度(Child-Pugh B)至重度(Child-Pugh C)肝损害患者,减少VITRAKVI的起始剂量。 储存:冷藏口服溶液在2°C到8°C (36°F 46°F)。不冻结。英文版说明书:
Vitrakvi(larotrectinib)Capsules and Oral SolutionU.S. Food and Drug Administration(FDA)today approved Vitrakvi(larotrectinib), the first ever oral TRK inhibitor, for the treatment of adult and pediatric patients with solid tumors that have a neurotrophic receptor tyrosine kinase(NTRK)gene fusion without a known acquired resistance mutation, are metastatic or where surgical resection is likely to result in severe morbidity, and have no satisfactory alternative treatments or that have progressed following treatment.This indication is approved under accelerated approval based on overall response rate(ORR)and duration of response(DOR).Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.Vitrakvi is the first treatment to receive a tumor-agnostic indication at the time of initial FDA approval. In clinical trials of patients with TRK fusion cancer, Vitrakvi demonstrated an ORR of 75 percent (N=55)(95% CI, 61%, 85%), including a 22 percent complete response(CR) rate.Clinical Trial ResultsThe FDA approval of Vitrakvi(larotrectinib)is based on pooled data across the Phase I adult trial, Phase II NAVIGATE trial and Phase I/II pediatric SCOUT trial (N=55).2 In pooled study results, Vitrakvi demonstrated an overall response rate(ORR)of 75 percent (95% CI, 61%, 85%) by blinded independent review committee (with 22 percent of patients achieving a complete response and 53 percent of patients achieving a partial response) across various tumor types, including soft tissue sarcoma, salivary gland, infantile fibrosarcoma, thyroid, lung, melanoma, colon, GIST, cholangiocarcinoma, appendix, breast and pancreas.2 Seventy-three percent of responding patients (N=41) had a duration of response (DOR) lasting six months or greater at the time of data cut-off.2 Median DOR (range 1.6+, 33.2+) and progression-free survival (PFS) had not been reached at the time of analysis.2,3 Median time to response was 1.84 months.3 In the safety database (N=176), which included patients with and without an NTRK gene fusion, the majority of adverse events (AEs) reported in greater than or equal to 10 percent of patients were grade 1 or 2.2 AEs of any grade observed in more than 20 percent of patients, regardless of attribution, included increased ALT(45%), increased AST(45%), anemia (42%), fatigue(37%), nausea(29%), dizziness (28%), cough (26%), vomiting(26%), constipation(23%), and diarrhea(22%).About Vitrakvi (larotrectinib)Vitrakvi is an oral TRK inhibitor for the treatment of adult and pediatric patients with solid tumors with an NTRK gene fusion without a known acquired resistance mutation that are either metastatic or where surgical resection will likely result in severe morbidity, and have no satisfactory alternative treatments or have progressed following treatment.This indication is approved under accelerated approval based on overall response rate and duration of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials. Research suggests that the NTRK genes can become abnormally fused to other genes, producing a TRK fusion protein that can lead to the growth and survival of solid tumors in various sites of the body.用药温馨提示:当您服用此药物时,需定期接受医疗专业人士的检查,以便随时针对其药效、副作用等情况进行监测。本网站所包含的信息旨在为患者提供帮助,不能代替医学建议和治疗。
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 拉罗替尼口服溶液larotrectinib(Vitrakvi Oral Solution 20mg/mL)说明书
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 拉罗替尼口服溶液larotrectinib(Vitrakvi Oral Solution 20mg/mL)说明书