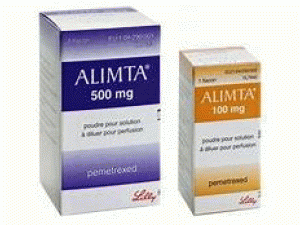
培美曲塞,培美曲塞二钠冻干粉注射剂(ALIMTA SDV 100MG 4ML)
 药店国别:无
产地国家:美国
处方药:是
所属类别: 100毫克 4毫升/瓶
包装规格: 100毫克 4毫升/瓶
计价单位:瓶
生产厂家中文参考译名:无
生产厂家英文名:ELI LILLY AND COMPANY
原产地英文商品名:ALIMTA SDV 100MG 4ML/Vial
原产地英文药品名:PEMETREXED DISODIUM
中文参考商品译名:ALIMTA冻干粉注射剂 100毫克 4毫升/瓶
中文参考药品译名:培美曲塞二钠
曾用名:力比泰,利比泰
药店国别:无
产地国家:美国
处方药:是
所属类别: 100毫克 4毫升/瓶
包装规格: 100毫克 4毫升/瓶
计价单位:瓶
生产厂家中文参考译名:无
生产厂家英文名:ELI LILLY AND COMPANY
原产地英文商品名:ALIMTA SDV 100MG 4ML/Vial
原产地英文药品名:PEMETREXED DISODIUM
中文参考商品译名:ALIMTA冻干粉注射剂 100毫克 4毫升/瓶
中文参考药品译名:培美曲塞二钠
曾用名:力比泰,利比泰
简介
近日,抗癌药ALIMTA(pemetrexed)被FDA批准与顺铂(另一种化疗药物)联合用于首次(初始)治疗晚期非鳞状非小细胞肺癌(NSCLC),这是一种已扩散的特定类型的NSCLC。ALIMTA(pemetrexed)被FDA批准作为单一药物(单独使用)用于晚期非鳞状非小细胞肺癌(NSCLC)患者的维持治疗。 2018年8月4日 公司:Eli LillyandALIMTA(培美曲塞 pemetrexed)注射,用于静脉注射。 美国最初批准:2004年 最近的重大变化适应症和用法:06/2018 剂量和用法:06/2018 作用机制 ALIMTA是一种叶酸类似物代谢抑制剂,可破坏对细胞至关重要的叶酸依赖性代谢过程复制。体外研究表明,培美曲塞可抑制胸苷酸合成酶(TS),二氢叶酸还原酶和甘氨酰胺核糖核苷酸甲酰转移酶(GARFT),它是从头参与的叶酸依赖性酶胸苷和嘌呤核苷酸的生物合成。 培美曲塞通过膜载体如细胞进入细胞减少叶酸载体和膜叶酸结合蛋白转运系统。一旦进入细胞,培美曲塞就会转化通过酶聚谷氨酸合成酶形成聚谷氨酸。 聚谷氨酸形式保留在细胞和细胞中是TS和GARFT的抑制剂。 适应症和用法 ALIMTA是一种叶酸类似物代谢抑制剂,表明:•与顺铂联合用于初期治疗局部晚期或转移性,非鳞状,非小细胞肺癌(NSCLC)的患者。 •与卡铂和pembrolizumab联合用于初步治疗转移性非鳞状NSCLC患者。该指征基于肿瘤应答率和无进展生存期在加速批准下获得批准。对该适应症的持续批准可能取决于确认试验中的临床益处的验证和描述。 •作为单一药物用于维持治疗局部晚期或转移的患者,非鳞状NSCLC剂量表明在四个铂类一线化疗周期后未发生进展。 •作为治疗复发,转移性非鳞状,NSCLC患者的单一药物,在事先化疗后。使用限制:ALIMTA不适用于治疗鳞状细胞,非小细胞肺癌的患者。 •初步治疗,与顺铂联合治疗,患有恶性胸膜间皮瘤的患者,其疾病无法切除或者不适合进行治愈性手术。 剂量和给药 •推荐剂量的ALIMTA,以单剂或顺铂或卡铂和pembrolizumab给药,肌酐清除率为45mL/分钟或更高的住院患者,每21天第1天10分钟内静脉输注500mg/m2-天周期。 •在第一剂ALIMTA开始前7天开始口服400mcg至1000mcg,每日一次,并在最后一剂ALIMTA后21天继续使用。 •肌肉注射维生素B12,1mg,第一剂ALIMTA前1周,每3个周期。 •给予地塞米松4 mg口服,每日两次,ALIMTA给药前一天,一天和一天。剂量形式和强度对于注射:在单剂量小瓶中100mg或500mg冻干粉末。 禁忌症 培美曲塞严重超敏反应史。警告和注意事项•骨髓抑制:可引起严重的骨髓抑制导致血细胞减少,并增加感染风险。当绝对中性粒细胞计数低于1500个细胞/mm3且血小板低于100,000个细胞/mm3时,不要给予ALIMTA。开始补充口服叶酸和肌内维生素B12,以减轻ALIMTA的血液和胃肠道毒性的严重程度。 •肾功能衰竭:可导致严重的,有时甚至是致命的肾功能衰竭。肌酐清除率小于45mL/min时不要给药。•大疱性和剥脱性皮肤毒性:永久性地停止严重和危及生命的大疱,水疱或去角质的皮肤毒性。 •间质性肺炎:保留急性发作的新的或进展不明原因的肺部症状。如果确认肺炎,则永久停用。 •放射回忆:可能发生在多年前接受过放射治疗的患者;永久停止放射线召回迹象。 •胚胎-胎儿毒性:可能导致胎儿伤害。告知患者胎儿的潜在风险并使用有效的避孕措施。 不良反应 •作为单一药物给药时,ALIMTA最常见的不良反应(发生率≥20%)是疲劳,恶心和厌食。 •当给予顺铂时,ALIMTA最常见的不良反应(发生率≥20%)有呕吐,中性粒细胞减少,贫血,口腔炎/咽炎,血小板减少和便秘。 •与卡铂和佩来珠单抗联合使用时,ALIMTA最常见的不良反应(发生率≥30%)是疲劳,恶心,便秘,皮疹,呕吐,呼吸困难,腹泻,头痛和食欲下降。药物相互作用布洛芬增加轻度过敏性肾功能损害患者的ALIMTA毒性风险。对于肌酐清除率在45mL/min和79mL/min之间的患者,修改布洛芬剂量。 用于特定人群使用注意:建议不要母乳喂养。 如何提供/存储和处理:如何提供ALIMTA,注射用培美曲塞,是一种白色至浅黄色或绿黄色冻干粉末,单剂量提供用于重建静脉输注的小瓶。NDC 0002-7640-01(VL7640):包含一(1)个单剂量小瓶100mg培美曲塞的纸箱。NDC 0002-7623-01(VL7623):包含一(1)500mg培美曲塞的单剂量小瓶的纸箱。存储和处理储存在25°C(77°F); 允许偏移15-30°C(59-86°F)[见USP受控室温]。ALIMTA是一种细胞毒性药物。 遵循适用的特殊处理和处理程序[见参考文献]。英文版说明
Medication nameGeneric name: Pemetrexed - InjectionPronunciation: (peh-meh-TREX-ed)Brand name(s): AlimtaUsesPemetrexed is used to treat certain types of cancer (e.g., lung cancer, mesothelioma). It is a chemotherapy drug that is used alone or in combination with other medications to slow or stop cancer cell growth.How to useThis medication comes with a Patient Information Leaflet available from your pharmacist. Read it carefully before you start using pemetrexed. If you have any questions regarding the information, consult your doctor or pharmacist.This medication is given by injection into a vein by a healthcare professional, usually over 10 minutes, once every 3 weeks.The dosage is based on your medical condition, body size, and response to therapy.To lower your chance of side effects, it is very important that you take folic acid vitamins and receive vitamin B12 shots before and during treatment with pemetrexed. Folic acid vitamins are available over-the-counter without a prescription, and folic acid can be found in many multivitamin products. Make sure your vitamin contains between 400 to 1000 micrograms (0.4 to 1 milligram) of folic acid. Take folic acid daily for at least 5 out of 7 days before your first dose of pemetrexed, during treatment, and for 3 weeks after your last treatment.Consult your doctor or pharmacist if you need help choosing a folic acid vitamin. Your doctor will give you a vitamin B12 shot into the muscle, usually 1 week before your first dose of pemetrexed and then once every 9 weeks during your treatment. Do not substitute oral vitamin B12 for the vitamin B12 shots. Consult your doctor or pharmacist for more details.To reduce your chance of having a skin reaction while using pemetrexed, your doctor will prescribe a corticosteroid medicine (e.g., dexamethasone) to take for a short period around the time of each treatment. Consult your doctor or pharmacist for more details.If this medication touches your skin, wash the skin immediately and completely with soap and water.PrecautionsBefore using pemetrexed, tell your doctor or pharmacist if you are allergic to it; or if you have any other allergies. This product may contain inactive ingredients, which can cause allergic reactions or other problems. Talk to your pharmacist for more details.This medication should not be used if you have certain medical conditions.Before using this medicine, consult your doctor or pharmacist if you have: severe kidney disease.Before using this medication, tell your doctor or pharmacist your medical history, especially of: decreased bone marrow function/blood cell disorders (e.g., anemia, leukopenia, thrombocytopenia), kidney problems, liver problems.Do not have immunizations/vaccinations without the consent of your doctor, and avoid contact with people who have recently received oral polio vaccine.Use caution with sharp objects like safety razors or nail cutters, and avoid activities such as contact sports to lower the chance of getting cut, bruised or injured.Wash your hands well to prevent the spread of infections.This drug may make you dizzy. Do not drive, use machinery, or do any activity that requires alertness until you are sure you can perform such activities safely.Limit alcoholic beverages.This medication is not recommended for use during pregnancy. It may cause harm to an unborn baby. Consult your doctor for more details and to discuss reliable forms of birth control. It is recommended that men and women use two effective forms of birth control (e.g., condoms, birth control pills) while using this medication and for some period afterwards.It is not known whether this drug passes into breast milk. Because of the potential risk to the infant, breast-feeding while using this drug is not recommended. Consult your doctor before breast-feeding.Drug interactionsDrug interactions may change how your medications work or increase your risk for serious side effects. This document does not contain all possible drug interactions. Keep a list of all the products you use (including prescription/nonprescription drugs and herbal products) and share it with your doctor and pharmacist. Do not start, stop, or change the dosage of any medicines without your doctor's approval.Some products that may interact with this drug include: probenecid, nonsteroidal anti-inflammatory drugs (NSAIDs such as aspirin, ibuprofen, naproxen).Low-dose aspirin, as prescribed by your doctor for specific medical reasons such as heart attack or stroke prevention (usually these dosages are 81-325 milligrams per day), should be continued. Consult your doctor or pharmacist for more details.Side effectsNausea, vomiting, loss of appetite, constipation, diarrhea, mouth sores, headache, body aches/pains, dizziness, and tiredness may occur. Nausea and vomiting can be severe. In some cases, drug therapy may be needed to prevent or relieve nausea and vomiting. Not eating before your treatment may help relieve vomiting. Changes in diet such as eating several small meals or limiting activity may help lessen some of these effects. If any of these effects persist or worsen, notify your doctor or pharmacist promptly.Temporary hair loss may occur. Normal hair growth should return after treatment has ended.Remember that your doctor has prescribed this medication because he or she has judged that the benefit to you is greater than the risk of side effects. Many people using this medication do not have serious side effects.Tell your doctor right away if you have any serious side effects, including: fast/pounding heartbeat, change in the amount of urine, dark urine, yellowing eyes/skin, severe stomach/abdominal pain, pain/redness/swelling of arms or legs, sore throat, painful/difficult swallowing, mental/mood changes, depression, numbness or tingling of the hands/feet, easy bruising/bleeding.Get medical help right away if any of these rare but very serious side effects occur: chest pain, new or worsening shortness of breath.This medication can lower the body's ability to fight an infection. Notify your doctor promptly if you develop any signs of an infection such as fever, chills or persistent sore throat.A very serious allergic reaction to this drug is unlikely, but get medical help right away if it occurs. Symptoms of a serious allergic reaction may include: rash, itching/swelling (especially of the face/tongue/throat), severe dizziness, trouble breathing.Pemetrexed can commonly cause a rash that is usually not serious and that can be prevented by taking corticosteroid medication (see How to Use section). However, if you do develop a rash, you may not be able to tell it apart from a rare rash that could be a sign of a severe reaction. Therefore, get medical help right away if you develop a rash.This is not a complete list of possible side effects. If you notice other effects not listed above, contact your doctor or pharmacist.In the US -Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.In Canada - Call your doctor for medical advice about side effects. You may report side effects to Health Canada at 1-866-234-2345.Missed doseFor the best benefits, it is important to receive each scheduled dose of this medication as directed. If you miss a dose, contact your doctor to establish a new dosing schedule.OverdoseIf overdose is suspected, contact a poison control center or emergency room immediately. US residents can call their local poison control center at 1-800-222-1222. Canada residents can call a provincial poison control center.NotesLaboratory and/or medical tests (e.g., complete blood counts, kidney and liver function tests) should be performed to monitor your progress or check for side effects. Consult your doctor for more details.Medical alertYour condition can cause complications in a medical emergency. For information about enrolling in MedicAlert, call 1-888-633-4298 (US) or 1-800-668-1507 (Canada).StorageNot applicable. This medication is given in a clinic and will not be stored at home.Photos by medication strengthClick the "Photos" link to see sample photographs for a specific medication strength.Common strengthsAlimta 100 mg IV SolutionAlimta 500 mg IV Solution*The photos shown are samples only. Not all photos of the drug may be displayed. Your medication may look different. If you have questions, ask your pharmacist.Important noteHOW TO USE THIS INFORMATION: This is a summary and does NOT have all possible information about this product. This information does not assure that this product is safe, effective, or appropriate for you. This information is not individual medical advice and does not substitute for the advice of your health care professional. Always ask your health care professional for complete information about this product and your specific health needs.Information last revised March 2013. Copyright(c) 2013 First DataBank, Inc.Selected from NATIONAL DRUG DATA FILE (NDDF) data included with permission and copyrighted by First DataBank, Inc., 2012. This copyrighted material has been downloaded from a licensed data provider.The above information is intended to supplement, not substitute for, the expertise and judgment of your health care professional. You should consult your health care professional before taking any drug, changing your diet, or commencing or discontinuing any course of treatment.用药温馨提示:当您服用此药物时,需定期接受医疗专业人士的检查,以便随时针对其药效、副作用等情况进行监测。本网站所包含的信息旨在为患者提供帮助,不能代替医学建议和治疗。
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 培美曲塞,培美曲塞二钠冻干粉注射剂(ALIMTA SDV 100MG 4ML)
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 培美曲塞,培美曲塞二钠冻干粉注射剂(ALIMTA SDV 100MG 4ML)