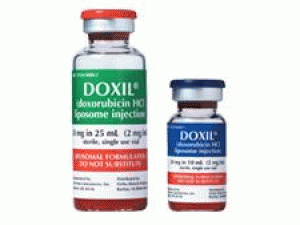
盐酸阿霉素脂质体注射溶液(doxorubicin HCl、Doxil 50mg/ml 25ml)说明书
产地国家:美国
 处 方 药:是
所属类别:20毫克/毫升 10毫升/瓶 1瓶
包装规格:20毫克/毫升 10毫升/瓶 1瓶
计价单位:瓶
生产厂家中文参考译名:JANSSEN PRODUCTS LP
生产厂家英文名:JANSSEN PRODUCTS LP
原产地英文商品名:DOXIL 2MG/ML 10ML SDV 1/EA
原产地英文药品名:DOXORUBICIN HCL LIPOSOME
中文参考商品译名:DOXIL注射溶液 20毫克/毫升 10毫升/瓶 1瓶
中文参考药品译名:盐酸阿霉素脂质体
处 方 药:是
所属类别:20毫克/毫升 10毫升/瓶 1瓶
包装规格:20毫克/毫升 10毫升/瓶 1瓶
计价单位:瓶
生产厂家中文参考译名:JANSSEN PRODUCTS LP
生产厂家英文名:JANSSEN PRODUCTS LP
原产地英文商品名:DOXIL 2MG/ML 10ML SDV 1/EA
原产地英文药品名:DOXORUBICIN HCL LIPOSOME
中文参考商品译名:DOXIL注射溶液 20毫克/毫升 10毫升/瓶 1瓶
中文参考药品译名:盐酸阿霉素脂质体

 处 方 药:是
所属类别:20毫克/毫升 10毫升/瓶 1瓶
包装规格:20毫克/毫升 10毫升/瓶 1瓶
计价单位:瓶
生产厂家中文参考译名:JANSSEN PRODUCTS LP
生产厂家英文名:JANSSEN PRODUCTS LP
原产地英文商品名:DOXIL 2MG/ML 10ML SDV 1/EA
原产地英文药品名:DOXORUBICIN HCL LIPOSOME
中文参考商品译名:DOXIL注射溶液 20毫克/毫升 10毫升/瓶 1瓶
中文参考药品译名:盐酸阿霉素脂质体
处 方 药:是
所属类别:20毫克/毫升 10毫升/瓶 1瓶
包装规格:20毫克/毫升 10毫升/瓶 1瓶
计价单位:瓶
生产厂家中文参考译名:JANSSEN PRODUCTS LP
生产厂家英文名:JANSSEN PRODUCTS LP
原产地英文商品名:DOXIL 2MG/ML 10ML SDV 1/EA
原产地英文药品名:DOXORUBICIN HCL LIPOSOME
中文参考商品译名:DOXIL注射溶液 20毫克/毫升 10毫升/瓶 1瓶
中文参考药品译名:盐酸阿霉素脂质体
简介:
近日,美国FDA批准DOXIL(doxorubicin HCl liposome injection,盐酸阿霉素脂质体)注射剂,治疗进展期及铂类化疗药化疗后复发的卵巢癌患者。 批准日期:1995年11月17日 公司:杨森制药 DOXIL(盐酸多柔比星脂质体[doxorubicin hydrochloride liposome])注射液,静脉使用 美国初次批准:1995年 警告:心肌肌病和与输注有关的反应 有关完整的盒装警告,请参阅完整的处方信息。 DOXIL可引起心肌损伤,包括急性左心衰竭。当蒽环类药物的累积剂量在450mg/m2至550mg/m2之间时,发生心肌病的风险为11%。在开始DOXIL之前,治疗期间和治疗之后评估左心室心脏功能。 可能发生严重,威胁生命的致命输液相关反应。11%的实体瘤患者发生了急性输注相关反应。拒绝DOXIL进行与输注相关的反应,并以降低的速度恢复。停止DOXIL输注以引起严重或危及生命的输注相关反应。 作用机理: DOXIL的活性成分是盐酸阿霉素。盐酸阿霉素的作用机理被认为与其结合DNA和抑制核酸合成的能力有关。细胞结构研究表明,细胞可以快速渗透并与核周染色质结合,对有丝分裂活性和核酸合成具有快速抑制作用,并可以诱变和诱使染色体畸变。 适应症和用途: DOXIL是一种蒽环类拓扑异构酶抑制剂,用于: 卵巢癌:铂类化学疗法失败后。 艾滋病相关的卡波济肉瘤:在先前的全身化学疗法失败或对该疗法不耐受之后。 多发性骨髓瘤:先前未曾接受过硼替佐米且已接受至少一种先前治疗的患者中与硼替佐米合用。 剂量和给药: 以1mg/min的初始速率管理DOXIL,以最大程度地减少输注反应的风险。如果没有发生与输注相关的反应,请在1小时内增加输注速率以完成给药。不要以推注或未稀释溶液的形式给药。 卵巢癌:每4周静脉输注50mg/m2。 艾滋病相关的卡波济肉瘤:每3周静脉注射20mg/m2。 多发性骨髓瘤:硼替佐米后第4天静脉输注30mg/m2。 剂量形式和强度:盐酸阿霉素脂质体注射液:单剂量小瓶中20mg/10mL(2mg/mL)和50mg/25mL(2mg/mL) 禁忌症:对盐酸阿霉素或DOXIL成分的超敏反应。 警告和注意事项: 手足综合症可能会发生。可能需要调整剂量或停药. 胚胎-胎儿毒性:可引起胎儿伤害。建议对胎儿的潜在风险。使用有效的避孕方法。 不良反应: 最常见的不良反应(>20%)为乏力,疲劳,发烧,厌食,恶心,呕吐,口腔炎,腹泻,便秘,手足综合征,皮疹,中性粒细胞减少,血小板减少和贫血。 要报告可疑的不良反应,请致电1-800-JANSSEN(1-800-526-7736)与Janssen Products,LP或致电1-800-FDA-1088或FDA联系www.fda.gov/medwatch。 在特定人口中使用:哺乳期:停止母乳喂养。 包装供应/存储和处理方式: DOXIL是无菌,半透明的红色脂质体分散液,溶于10毫升或30毫升玻璃单剂量小瓶中。 提供以下单独包装的小瓶: 将未打开的DOXIL小瓶在2°C至8°C(36°F至46°F)的温度下冷藏。 不要冻结。 丢弃未使用的部分。 DOXIL是一种细胞毒性药物。 请遵循适用的特殊处理和处置程序。 英文说明书: IMPORTANT SAFETY INFORMATION: Serious and possibly permanent heart-related side effects that may lead to congestive heart failure can occur in patients treated with DOXIL®. Inform your doctor of any history of heart disease, radiation to your chest, or prior anticancer therapy. Your doctor will monitor your heart function. Infusion reactions may also occur during administration. Be sure to tell your doctor if you have any symptoms during infusion, including: flushing, shortness of breath, facial swelling, headaches, chills, chest pain, back pain, tightness in your chest or throat, dizziness, lightheadedness, low blood pressure, fever, increased heart rate, itchiness, rash, blue-colored skin, fainting, wheezing, and breathing problems. In some cases, these reactions may be serious and sometimes life-threatening and may be fatal. Your healthcare provider will temporarily stop the infusion to control the symptoms and resume treatment at a reduced rate. Treatment with DOXIL® will be stopped for serious or life-threatening infusion-related reactions. INDICATIONS: DOXIL® (doxorubicin HCl liposome injection) is indicated for the treatment of patients with ovarian cancer whose disease has progressed or recurred after platinum-based chemotherapy. DOXIL® is indicated for the treatment of AIDS-related Kaposi's sarcoma in patients after failure of prior systemic chemotherapy or intolerance to such therapy. DOXIL®, in combination with bortezomib, is indicated for the treatment of patients with multiple myeloma who have not previously received bortezomib and have received at least one prior therapy. DOXIL® is administered intravenously by your healthcare professional. Doxil (Doxorubicin Hcl Liposome Injection) You should not take DOXIL® if you have a prior history of allergic reactions to doxorubicin or other ingredients found in the formulation. Please inform your doctor about your history of allergic reactions to medications or other substances. DOXIL® may severely reduce the number of blood cells (red blood cells, white blood cells, and cells that prevent bleeding called platelets) in your body that may potentially increase risk of infections, anemia, and bleeding. Speak to your doctor if you notice any changes in your health such as a new onset fever or symptoms of infection. Your doctor will monitor your blood laboratory results. Hand-Foot Syndrome may occur while taking DOXIL®. This may lead to tingling or burning, redness, flaking, bothersome swelling, small blisters, or small sores on palms of hands or soles of feet. In certain cases, this reaction can be more severe leading to serious infections, interfering with walking and other daily activities. Cases of oral cancer have been reported in people who had taken DOXIL® for more than one year. The oral cancer was diagnosed during treatment and up to 6 years after the last dose. Your doctor will examine you at regular times for the signs and symptoms of oral cancer. If you are pregnant, planning to become pregnant, or breastfeeding, inform your doctor. DOXIL® can cause fetal harm. Breastfeeding should be discontinued during treatment with DOXIL®. Females and males of reproductive potential should use effective contraception during and for 6 months following treatment with DOXIL®. DOXIL® may cause temporary or permanent infertility. The most common side effects reported in at least 20% of patients treated with DOXIL® during clinical studies were: weakness, tiredness, fever, nausea, stomatitis (painful redness, swelling, or sores in the mouth), vomiting, diarrhea, constipation, loss of appetite, hand-foot syndrome, rash, low white blood cell count, low platelet count, and anemia. Tell your doctor if you experience these or other side effects. In the treatment of multiple myeloma, nerve damage called peripheral neuropathy, which may lead to pain, numbness, burning sensation, tingling, and more serious symptoms, was reported in >40% of patients. Be sure to tell your doctor immediately if you experience any of these or other symptoms. Following administration, DOXIL® may turn urine and other bodily fluids a reddish-orange color. This is due to the color of DOXIL® and will go away as the drug leaves the body. Talk to your doctor if you have a history of heart disease or liver disease, or have received prior radiation therapy and/or anticancer therapy.用药温馨提示:当您服用此药物时,需定期接受医疗专业人士的检查,以便随时针对其药效、副作用等情况进行监测。本网站所包含的信息旨在为患者提供帮助,不能代替医学建议和治疗。
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 盐酸阿霉素脂质体注射溶液(doxorubicin HCl、Doxil 50mg/ml 25ml)说明书
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 盐酸阿霉素脂质体注射溶液(doxorubicin HCl、Doxil 50mg/ml 25ml)说明书